Inhibition of the FAD containing ER oxidoreductin 1 (Ero1) protein by EN-460 as a strategy for treatment of multiple myeloma
- PMID: 30850265
- PMCID: PMC6554731
- DOI: 10.1016/j.bmc.2019.02.016
Inhibition of the FAD containing ER oxidoreductin 1 (Ero1) protein by EN-460 as a strategy for treatment of multiple myeloma
Abstract
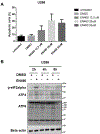
Multiple myeloma (MM) cells demonstrate high basal endoplasmic reticulum (ER) stress and are typically exquisitely sensitive to agents such as proteasome inhibitors that activate the unfolded protein response. The flavin adenosine dinucleotide (FAD) containing endoplasmic reticulum oxidoreductin enzyme (Ero1L) catalyzes de-novo disulfide bridge formation of ER resident proteins and contributes to proper protein folding. Here we show that increased Ero1L expression is prognostic of poor outcomes for MM patients relapsing on therapy. We propose that targeting protein folding via inhibition of Ero1L may represent a novel therapeutic strategy for the treatment of MM. In this report we show that treatment of MM cells with EN-460, a known inhibitor of ERO1L, was sufficient to inhibit cell proliferation and induce apoptosis. Furthermore, we show that cell death correlated in part with induction of ER stress. We also show that EN460 inhibited the enzyme activity of Ero1L, with an IC50 of 22.13 μM, consistent with previous reports. However, EN-460 was also found to inhibit other FAD-containing enzymes including MAO-A (IC50 = 7.91 μM), MAO-B (IC50 = 30.59 μM) and LSD1 (IC50 = 4.16 μM), suggesting overlap in inhibitor activity and the potential need to develop more specific inhibitors to enable pharmacological validation of ERO1L as a target for the treatment of MM. We additionally prepared and characterized azide-tagged derivatives of EN-460 as possible functional probe compounds (e.g., for photo-affinity labeling) for future target-engagement studies and further development of structure-activity relationships.
Keywords: Flavin adenosine dinucleotide; Oxidative stress; Unfolded protein response.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures




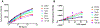



References
-
- Anderson KC, Kyle RA, Dalton WS, Landowski T, Shain K, Jove R, Hazlehurst L, Berenson J, Multiple Myeloma: New Insights and Therapeutic Approaches, Hematology (Am Soc Hematol Educ Program), (2000) 147–165. - PubMed
-
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC, The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells, Cancer research, 61 (2001) 3071–3076. - PubMed
-
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FW, Chanan-Khan AA, Orlowski RZ, Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma, Blood, 110 (2007) 3281–3290. - PMC - PubMed
-
- Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG, The proteasome and proteasome inhibitors in multiple myeloma, Cancer Metastasis Rev, 36 (2017) 561–584. - PubMed
-
- Wallington-Beddoe CT, Sobieraj-Teague M, Kuss BJ, Pitson SM, Resistance to proteasome inhibitors and other targeted therapies in myeloma, Br J Haematol, 182 (2018) 11–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Research Materials

