68Ga-DOTA-E[c(RGDfK)]2 PET Imaging of SHARPIN-Regulated Integrin Activity in Mice
- PMID: 30850498
- PMCID: PMC6785786
- DOI: 10.2967/jnumed.118.222026
68Ga-DOTA-E[c(RGDfK)]2 PET Imaging of SHARPIN-Regulated Integrin Activity in Mice
Abstract
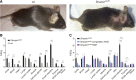
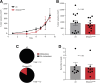
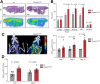
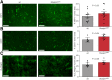
Shank-associated RH domain-interacting protein (SHARPIN) is a cytosolic protein that plays a key role in activation of nuclear factor κ-light-chain enhancer of activated B cells and regulation of inflammation. Furthermore, SHARPIN controls integrin-dependent cell adhesion and migration in several normal and malignant cell types, and loss of SHARPIN correlates with increased integrin activity in mice. Arginyl-glycyl-aspartic acid (RGD), a cell adhesion tripeptide motif, is an integrin recognition sequence that facilitates PET imaging of integrin upregulation during tumor angiogenesis. We hypothesized that increased integrin activity due to loss of SHARPIN protein would affect the uptake of αvβ3-selective cyclic, dimeric peptide 68Ga-DOTA-E[c(RGDfK)]2, where E[c(RGDfk)]2 = glutamic acid-[cyclo(arginyl-glycyl-aspartic acid-D-phenylalanine-lysine)], both in several tissue types and in the tumor microenvironment. To test this hypothesis, we used RGD-based in vivo PET imaging to evaluate wild-type (wt) and SHARPIN-deficient mice (Sharpincpdm , where cpdm = chronic proliferative dermatitis in mice) with and without melanoma tumor allografts. Methods:Sharpincpdm mice with spontaneous null mutation in the Sharpin gene and their wt littermates with or without B16-F10-luc melanoma tumors were studied by in vivo imaging and ex vivo measurements with cyclic-RGD peptide 68Ga-DOTA-E[c(RGDfK)]2 After the last 68Ga-DOTA-E[c(RGDfK)]2 peptide PET/CT, tumors were cut into cryosections for autoradiography, histology, and immunohistochemistry. Results: The ex vivo uptake of 68Ga-DOTA-E[c(RGDfK)]2 in the mouse skin and tumor was significantly higher in Sharpincpdm mice than in wt mice. B16-F10-luc tumors were detected 4 d after inoculation, without differences in volume or blood flow between the mouse strains. PET imaging with 68Ga-DOTA-E[c(RGDfK)]2 peptide at day 10 after inoculation revealed significantly higher uptake in the tumors transplanted into Sharpincpdm mice than in wt mice. Furthermore, tumor vascularization was increased in the Sharpincpdm mice. Conclusion:Sharpincpdm mice demonstrated increased integrin activity and vascularization in B16-F10-luc melanoma tumors, as demonstrated by RGD-based in vivo PET imaging. These data indicate that SHARPIN, a protein previously associated with increased cancer growth and metastasis, may also have important regulatory roles in controlling the tumor microenvironment.
Keywords: PET; RGD; SHARPIN; melanoma; αvβ3 integrin.
© 2019 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
References
-
- Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14:430–442. - PubMed
-
- Pfaff M, Tangemann K, Muller B, et al. Selective recognition of cyclic RGD peptides of NMR defined conformation by αIIbβ3, αVβ3, and α5β1 integrins. J Biol Chem. 1994;269:20233–20238. - PubMed
-
- Zannetti A, Del Vecchio S, Iommelli F, et al. Imaging of αvβ3 expression by a bifunctional chimeric RGD peptide not cross-reacting with αvβ5. Clin Cancer Res. 2009;15:5224–5233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
