Crystal structure of human mitochondrial trifunctional protein, a fatty acid β-oxidation metabolon
- PMID: 30850536
- PMCID: PMC6442613
- DOI: 10.1073/pnas.1816317116
Crystal structure of human mitochondrial trifunctional protein, a fatty acid β-oxidation metabolon
Abstract
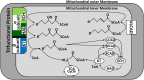
Membrane-bound mitochondrial trifunctional protein (TFP) catalyzes β-oxidation of long chain fatty acyl-CoAs, employing 2-enoyl-CoA hydratase (ECH), 3-hydroxyl-CoA dehydrogenase (HAD), and 3-ketothiolase (KT) activities consecutively. Inherited deficiency of TFP is a recessive genetic disease, manifesting in hypoketotic hypoglycemia, cardiomyopathy, and sudden death. We have determined the crystal structure of human TFP at 3.6-Å resolution. The biological unit of the protein is α2β2 The overall structure of the heterotetramer is the same as that observed by cryo-EM methods. The two β-subunits make a tightly bound homodimer at the center, and two α-subunits are bound to each side of the β2 dimer, creating an arc, which binds on its concave side to the mitochondrial innermembrane. The catalytic residues in all three active sites are arranged similarly to those of the corresponding, soluble monofunctional enzymes. A structure-based, substrate channeling pathway from the ECH active site to the HAD and KT sites is proposed. The passage from the ECH site to the HAD site is similar to those found in the two bacterial TFPs. However, the passage from the HAD site to the KT site is unique in that the acyl-CoA intermediate can be transferred between the two sites by passing along the mitochondrial inner membrane using the hydrophobic nature of the acyl chain. The 3'-AMP-PPi moiety is guided by the positively charged residues located along the "ceiling" of the channel, suggesting that membrane integrity is an essential part of the channel and is required for the activity of the enzyme.
Keywords: fatty acid oxidation; metabolon; mitochondrial trifunctional protein; substrate channeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

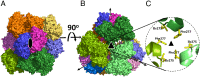
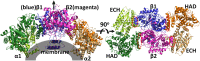

References
-
- Kunau WH, Dommes V, Schulz H. Beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: A century of continued progress. Prog Lipid Res. 1995;34:267–342. - PubMed
-
- Eaton S, et al. The mitochondrial trifunctional protein: Centre of a beta-oxidation metabolon? Biochem Soc Trans. 2000;28:177–182. - PubMed
-
- Spiekerkoetter U, Khuchua Z, Yue Z, Bennett MJ, Strauss AW. General mitochondrial trifunctional protein (TFP) deficiency as a result of either alpha- or beta-subunit mutations exhibits similar phenotypes because mutations in either subunit alter TFP complex expression and subunit turnover. Pediatr Res. 2004;55:190–196. - PubMed
-
- Ibdah JA, Yang Z, Bennett MJ. Liver disease in pregnancy and fetal fatty acid oxidation defects. Mol Genet Metab. 2000;71:182–189. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

