RNA-seq of Isolated Chromaffin Cells Highlights the Role of Sex-Linked and Imprinted Genes in Adrenal Medulla Development
- PMID: 30850723
- PMCID: PMC6408553
- DOI: 10.1038/s41598-019-40501-0
RNA-seq of Isolated Chromaffin Cells Highlights the Role of Sex-Linked and Imprinted Genes in Adrenal Medulla Development
Abstract
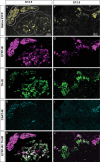
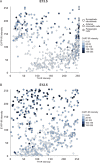
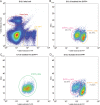
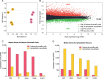
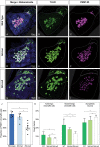
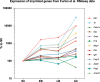
Adrenal chromaffin cells and sympathetic neurons synthesize and release catecholamines, and both cell types are derived from neural crest precursors. However, they have different developmental histories, with sympathetic neurons derived directly from neural crest precursors while adrenal chromaffin cells arise from neural crest-derived cells that express Schwann cell markers. We have sought to identify the genes, including imprinted genes, which regulate the development of the two cell types in mice. We developed a method of separating the two cell types as early as E12.5, using differences in expression of enhanced yellow fluorescent protein driven from the tyrosine hydroxylase gene, and then used RNA sequencing to confirm the characteristic molecular signatures of the two cell types. We identified genes differentially expressed by adrenal chromaffin cells and sympathetic neurons. Deletion of a gene highly expressed by adrenal chromaffin cells, NIK-related kinase, a gene on the X-chromosome, results in reduced expression of adrenaline-synthesizing enzyme, phenyl-N-methyl transferase, by adrenal chromaffin cells and changes in cell cycle dynamics. Finally, many imprinted genes are up-regulated in chromaffin cells and may play key roles in their development.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Differences in CART expression and cell cycle behavior discriminate sympathetic neuroblast from chromaffin cell lineages in mouse sympathoadrenal cells.Dev Neurobiol. 2016 Feb;76(2):137-49. doi: 10.1002/dneu.22304. Epub 2015 Jun 1. Dev Neurobiol. 2016. PMID: 25989220 Free PMC article.
-
Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla.Stem Cells. 2009 Oct;27(10):2602-13. doi: 10.1002/stem.180. Stem Cells. 2009. PMID: 19609938
-
Lineage and stage specific requirement for Dicer1 in sympathetic ganglia and adrenal medulla formation and maintenance.Dev Biol. 2015 Apr 15;400(2):210-23. doi: 10.1016/j.ydbio.2015.01.026. Epub 2015 Feb 4. Dev Biol. 2015. PMID: 25661788
-
Generation of neuroendocrine chromaffin cells from sympathoadrenal progenitors: beyond the glucocorticoid hypothesis.Ann N Y Acad Sci. 2002 Oct;971:554-9. doi: 10.1111/j.1749-6632.2002.tb04526.x. Ann N Y Acad Sci. 2002. PMID: 12438182 Review.
-
The development of the chromaffin cell lineage from the neural crest.Auton Neurosci. 2009 Nov 17;151(1):10-6. doi: 10.1016/j.autneu.2009.07.020. Epub 2009 Aug 14. Auton Neurosci. 2009. PMID: 19683477 Review.
Cited by
-
BET and CDK Inhibition Reveal Differences in the Proliferation Control of Sympathetic Ganglion Neuroblasts and Adrenal Chromaffin Cells.Cancers (Basel). 2022 Jun 1;14(11):2755. doi: 10.3390/cancers14112755. Cancers (Basel). 2022. PMID: 35681734 Free PMC article.
-
NEDD4 Promotes Sertoli Cell Proliferation and Adult Leydig Cell Differentiation in the Murine Testis.Endocrinology. 2025 Jul 8;166(9):bqaf115. doi: 10.1210/endocr/bqaf115. Endocrinology. 2025. PMID: 40605617 Free PMC article.
-
Early transcriptomic response of mouse adrenal gland and Y-1 cells to dexamethasone.Endocr Connect. 2022 Jul 25;11(8):e220064. doi: 10.1530/EC-22-0064. Print 2022 Aug 1. Endocr Connect. 2022. PMID: 35904237 Free PMC article.
-
Single-cell transcriptomic analyses provide insights into the developmental origins of neuroblastoma.Nat Genet. 2021 May;53(5):683-693. doi: 10.1038/s41588-021-00806-1. Epub 2021 Mar 25. Nat Genet. 2021. PMID: 33767450
-
Identification and functional activity of Nik related kinase (NRK) in benign hyperplastic prostate.J Transl Med. 2024 Mar 9;22(1):255. doi: 10.1186/s12967-024-05048-3. J Transl Med. 2024. PMID: 38459501 Free PMC article.
References
-
- Landis SC, Patterson PH. Neural crest cell lineages. Trends Neurosci. 1981;4:172–175. doi: 10.1016/0166-2236(81)90056-4. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

