Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways
- PMID: 30851061
- PMCID: PMC6536408
- DOI: 10.1111/cpr.12579
Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways
Erratum in
-
Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways.Cell Prolif. 2020 Oct;53(10):e12775. doi: 10.1111/cpr.12775. Cell Prolif. 2020. PMID: 33079421 Free PMC article. No abstract available.
Abstract
Objectives: Neutrophils are thought to release neutrophil extracellular traps (NETs) to form in response to exogenous bacteria, viruses and other pathogens. However, the mechanisms underlying NET formation during sterile inflammation are still unclear. In this study, we would like to identify neutrophil extracellular traps formation during sterile inflammation and tissue injury and associated pathways and its mechanism.
Materials and methods: We identified different injuries such as chemical-induced and trauma-induced formation of NETs and investigated mechanism of the formation of NETs in vitro and in vivo during the treatment of mtDNA.
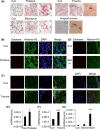
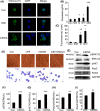
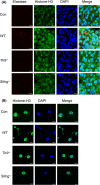
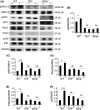
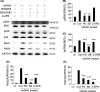
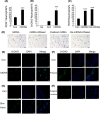
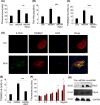
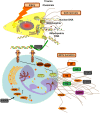
Results: Here, we find the release of mitochondrial DNA (mtDNA) and oxidized mtDNA in acute peripheral tissue trauma models or other chemically induced lung injury, and moreover, endogenous mtDNA and oxidized mtDNA induce the formation of NETs and sterile inflammation. Oxidized mtDNA is a more potent inducer of NETs. Mitochondrial DNA activates neutrophils via cyclic GMP-AMP synthase (cGAS)-STING and the Toll-like receptor 9 (TLR9) pathways and increases the production of neutrophil elastase and extracellular neutrophil-derived DNA in NETs. Mitochondrial DNA also increases the production of reactive oxygen species (ROS) and expression of the NET-associated proteins Rac 2 and peptidylarginine deiminase 4 (PAD4).
Conclusions: Altogether, these findings highlight that endogenous mitochondrial DNA inducted NETs formation and subsequent sterile inflammation and the mechanism associated with NET formation.
Keywords: Toll-like receptor 9; cyclic GMP-AMP synthase; mitochondrial DNA; neutrophil extracellular traps.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

References
-
- Brinkmann V, Reichard U, Goosmann C, et al. neutrophil extracellular traps kill bacteria. Science. 2004;303:1532‐1535. - PubMed
-
- Allam R, Kumar SV, Darisipudi MN, et al. Extracellular histones in tissue injury and inflammation. J Mol Med. 2014;92:465‐472. - PubMed
-
- Yousefi S, Mihalache C, Kozlowski E, et al. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438‐1444. - PubMed
-
- McIlroy DJ, Jarnicki AG, Au GG, et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. J Crit Care. 2014;29(1133):e1131‐1135. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

