Randomized study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: Pre-specified analysis of the Chinese population from the BERSON clinical trial
- PMID: 30851062
- PMCID: PMC6594089
- DOI: 10.1111/dom.13700
Randomized study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: Pre-specified analysis of the Chinese population from the BERSON clinical trial
Abstract
Aim: The aim of this study was to evaluate the efficacy and safety of evolocumab with background atorvastatin in Chinese patients with type 2 diabetes mellitus (T2DM) and hyperlipidaemia or mixed dyslipidaemia.
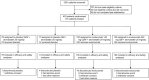
Materials and methods: This is a pre-specified analysis of patients in the BERSON study (ClinicalTrials.gov, NCT02662569) in China. Patients initiated background atorvastatin 20 mg/d, after which they were randomized 2:2:1:1 to evolocumab 140 mg every 2 weeks (Q2W) or 420 mg monthly (QM) or to placebo Q2W or QM. Co-primary endpoints were percentage change in LDL cholesterol (LDL-C) from baseline to week 12 and from baseline to the mean of weeks 10 and 12. Additional endpoints included atherogenic lipids, glycaemic measures and adverse events (AEs).
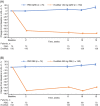
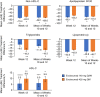
Results: Among 453 patients randomized in China, 451 received at least one dose of study drug (evolocumab or placebo). Evolocumab significantly reduced LDL-C compared with placebo at week 12 (Q2W, -85.0%; QM, -74.8%) and at the mean of weeks 10 and 12 (Q2W, -80.4%; QM, -81.0%) (adjusted P < 0.0001 for all) when administered with background atorvastatin. Non-HDL-C, ApoB100, total cholesterol, Lp(a), triglycerides, HDL-C and VLDL-C significantly improved with evolocumab vs placebo. No new safety findings were observed with evolocumab. The incidence of diabetes AEs was higher with evolocumab compared with placebo. There were no differences over time between evolocumab and placebo in measures of glycaemic control.
Conclusions: In patients in China with T2DM and hyperlipidaemia or mixed dyslipidaemia receiving background atorvastatin, evolocumab significantly reduced LDL-C and other atherogenic lipids, was well tolerated, and had no notable impact on glycaemic measures.
Keywords: dyslipidaemia; evolocumab; hyperlipidaemia; phase 3; type 2 diabetes.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
F. G. E. has served as a speaker for and has received grants for research from Amgen Inc., Sanofi, Boehringer, Eli Lilly, Novo Nordisk and AstraZeneca. A. J. L. has served as an advisory board and steering committee member for and has received research grants and speaker fees from Amgen Inc. M. L. M., N. W. and A. W. H. are employed by and own stock in Amgen Inc. Y. C., Z. Y., J. L. and J. G. have no conflicts of interest to disclose.
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 8th ed.: The Atlas; 2017. http://www.idf.org. Accessed April 3, 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

