Serum antibody profiles in individuals with latent Mycobacterium tuberculosis infection
- PMID: 30851131
- PMCID: PMC6767593
- DOI: 10.1111/1348-0421.12674
Serum antibody profiles in individuals with latent Mycobacterium tuberculosis infection
Abstract
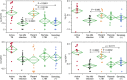
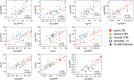
One-third of the world's humans has latent tuberculosis infection (LTBI), representing a large pool of potentially active TB. Recent LTBI carries a higher risk of disease progression than remote LTBI. Recent studies suggest important roles of antibodies in TB pathology, prompting us to investigate serum antibody profiles in a cohort with LTBI. In this single-center prospective observational study, we analyzed IgG-antibody concentrations against five major Mycobacterium tuberculosis (Mtb) antigens (including 6 kDa early secretory antigenic target (ESAT6), CFP10, and antigen 85A, which are expressed mainly in the growth phase; and mycobacterial DNA-binding protein 1 (MDP1) and alpha-crystallin like protein (Acr), which are expressed in the dormant phases) in individuals with recent (n=13) or remote (n=12) LTBI, no Mtb infection (n=19), or active TB (n=15). Antibody titers against ESAT6 and MDP1 were significantly higher in individuals with recent LTBI than in those with no Mtb infection or remote LTBI. All pairwise antibody titers against these five major antigens were significantly correlated throughout the stages of Mtb infection. Five individuals with recent LTBI had significantly higher antibody titers against ESAT6 (P = 0.03), Ag85A (P = 0.048), Acr (P = 0.057), and MDP1 (P = 0.0001) than in individuals with remote LTBI; they were also outside the normal range (+2 SDs). One of these individuals was diagnosed with active pulmonary TB at 18-month follow-up examination. These findings indicated that concentrations of antibodies against both multiplying and dormant Mtb are higher in recent LTBI and that individuals with markedly higher antibody titers may be appropriate candidates for prophylactic therapy.
Keywords: Biomarker; latent tuberculosis infection; mycobacterial DNA-binding protein 1; serum antibody.
© 2019 The Societies and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures


Similar articles
-
IgG subclasses' response to a set of mycobacterial antigens in different stages of Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2018 Jan;108:70-76. doi: 10.1016/j.tube.2017.10.010. Epub 2017 Oct 31. Tuberculosis (Edinb). 2018. PMID: 29523330
-
Antigen 85A and mycobacterial DNA-binding protein 1 are targets of immunoglobulin G in individuals with past tuberculosis.Microbiol Immunol. 2013 Jan;57(1):30-7. doi: 10.1111/j.1348-0421.2012.12005.x. Microbiol Immunol. 2013. PMID: 23157580
-
Difference in Antibody Responses to Mycobacterium tuberculosis Antigens in Japanese Tuberculosis Patients Infected with the Beijing/Non-Beijing Genotype.J Immunol Res. 2017;2017:4797856. doi: 10.1155/2017/4797856. Epub 2017 Jan 15. J Immunol Res. 2017. PMID: 28182078 Free PMC article.
-
Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination.Semin Immunol. 2018 Oct;39:88-101. doi: 10.1016/j.smim.2018.07.001. Epub 2018 Jul 7. Semin Immunol. 2018. PMID: 30327124 Review.
-
Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis.Vaccine. 2014 Feb 3;32(6):712-6. doi: 10.1016/j.vaccine.2013.11.065. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24300592 Review.
Cited by
-
Advancing personalized medicine for tuberculosis through the application of immune profiling.Front Cell Infect Microbiol. 2023 Feb 10;13:1108155. doi: 10.3389/fcimb.2023.1108155. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36844400 Free PMC article. Review.
-
Humoral correlate of vaccine-mediated protection from tuberculosis identified in humans and non-human primates.bioRxiv [Preprint]. 2024 Dec 9:2024.12.05.627012. doi: 10.1101/2024.12.05.627012. bioRxiv. 2024. PMID: 39713388 Free PMC article. Preprint.
-
Mycobacterium tuberculosis: immune response, biomarkers, and therapeutic intervention.MedComm (2020). 2024 Jan 6;5(1):e419. doi: 10.1002/mco2.419. eCollection 2024 Jan. MedComm (2020). 2024. PMID: 38188605 Free PMC article. Review.
-
Serodiagnosis of M. abscessus species pulmonary disease using anti-glycopeptidolipid-core IgA antibody.IJTLD Open. 2024 Dec 1;1(12):575-577. doi: 10.5588/ijtldopen.24.0441. eCollection 2024 Dec. IJTLD Open. 2024. PMID: 39679209 Free PMC article. No abstract available.
-
Cytokine and Chemokine Responses of Peripheral Blood Mononuclear Cells from Dogs Infected with Mycobacterium bovis.Pathogens. 2024 Dec 30;14(1):17. doi: 10.3390/pathogens14010017. Pathogens. 2024. PMID: 39860978 Free PMC article.
References
-
- World Health Organization. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016.
-
- Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;61:1376‐1395. - PubMed
-
- Horsburgh CR, Jr. , Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364:1441‐1448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

