Predictive model of spread of Parkinson's pathology using network diffusion
- PMID: 30851444
- PMCID: PMC7180066
- DOI: 10.1016/j.neuroimage.2019.03.001
Predictive model of spread of Parkinson's pathology using network diffusion
Abstract
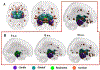
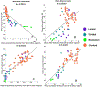
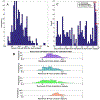
Growing evidence suggests that a "prion-like" mechanism underlies the pathogenesis of many neurodegenerative disorders, including Parkinson's disease (PD). We extend and tailor previously developed quantitative and predictive network diffusion model (NDM) to PD, by specifically modeling the trans-neuronal spread of alpha-synuclein outward from the substantia nigra (SN). The model demonstrated the spatial and temporal patterns of PD from neuropathological and neuroimaging studies and was statistically validated using MRI deformation of 232 Parkinson's patients. After repeated seeding simulations, the SN was found to be the most likely seed region, supporting its unique lynchpin role in Parkinson's pathology spread. Other alternative spread models were also evaluated for comparison, specifically, random spread and distance-based spread; the latter tests for Braak's original caudorostral transmission theory. We showed that the distance-based spread model is not as well supported as the connectivity-based model. Intriguingly, the temporal sequencing of affected regions predicted by the model was in close agreement with Braak stages III-VI, providing what we consider a "computational Braak" staging system. Finally, we investigated whether the regional expression patterns of implicated genes contribute to regional atrophy. Despite robust evidence for genetic factors in PD pathogenesis, NDM outperformed regional genetic expression predictors, suggesting that network processes are far stronger mediators of regional vulnerability than innate or cell-autonomous factors. This is the first finding yet of the ramification of prion-like pathology propagation in Parkinson's, as gleaned from in vivo human imaging data. The NDM is potentially a promising robust and clinically useful tool for diagnosis, prognosis and staging of PD.
Keywords: Network diffusion; Parkinson's disease; Prions; Substantia nigra; Synuclein.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
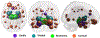






Similar articles
-
α-Synuclein: the long distance runner.Brain Pathol. 2013 May;23(3):350-7. doi: 10.1111/bpa.12046. Brain Pathol. 2013. PMID: 23587141 Free PMC article. Review.
-
Regional transcriptional architecture of Parkinson's disease pathogenesis and network spread.Brain. 2019 Oct 1;142(10):3072-3085. doi: 10.1093/brain/awz223. Brain. 2019. PMID: 31359041 Free PMC article.
-
Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson's disease?Expert Rev Neurother. 2012 Jun;12(6):673-86. doi: 10.1586/ern.12.47. Expert Rev Neurother. 2012. PMID: 22650170 Review.
-
Modeling Lewy pathology propagation in Parkinson's disease.Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1(0 1):S85-7. doi: 10.1016/S1353-8020(13)70022-1. Parkinsonism Relat Disord. 2014. PMID: 24262196 Free PMC article. Review.
-
Neuropathology of α-synuclein propagation and braak hypothesis.Mov Disord. 2016 Feb;31(2):152-60. doi: 10.1002/mds.26421. Epub 2015 Sep 4. Mov Disord. 2016. PMID: 26340605 Review.
Cited by
-
Connectome-based biophysics models of Alzheimer's disease diagnosis and prognosis.Transl Res. 2023 Apr;254:13-23. doi: 10.1016/j.trsl.2022.08.008. Epub 2022 Aug 27. Transl Res. 2023. PMID: 36031051 Free PMC article. Review.
-
Contrastive machine learning reveals Parkinson's disease specific features associated with disease severity and progression.Commun Biol. 2024 Aug 7;7(1):954. doi: 10.1038/s42003-024-06648-x. Commun Biol. 2024. PMID: 39112797 Free PMC article.
-
Hubs of long-distance co-alteration characterize brain pathology.Hum Brain Mapp. 2020 Oct 1;41(14):3878-3899. doi: 10.1002/hbm.25093. Epub 2020 Jun 20. Hum Brain Mapp. 2020. PMID: 32562581 Free PMC article.
-
Combined Model of Aggregation and Network Diffusion Recapitulates Alzheimer's Regional Tau-Positron Emission Tomography.Brain Connect. 2021 Oct;11(8):624-638. doi: 10.1089/brain.2020.0841. Epub 2021 Jul 16. Brain Connect. 2021. PMID: 33947253 Free PMC article.
-
Modeling seeding and neuroanatomic spread of pathology in amyotrophic lateral sclerosis.Neuroimage. 2022 May 1;251:118968. doi: 10.1016/j.neuroimage.2022.118968. Epub 2022 Feb 7. Neuroimage. 2022. PMID: 35143975 Free PMC article.
References
-
- Poewe WH, Wenning GK. The natural history of Parkinson’s disease. Ann Neurol. 2006;44(3 Suppl 1):VII2–I6. - PubMed
-
- Jellinger KA. Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution--a pilot study. Acta Neuropathol [Internet] 2003. September [cited 2013 Apr 15];106(3):191–201. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12845452 - PubMed
-
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging [Internet] 2003. [cited 2012 Nov 13];24(2):197–211. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12498954 - PubMed
-
- Del Tredici K, Braak H. Review: Sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol [Internet] 2016. February;42(1):33–50. Available from: http://doi.wiley.com/10.1111/nan.12298 - DOI - PubMed
-
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science (80- ) [Internet] 2012. November 15 [cited 2012 Nov 15];338(6109):949–53. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1227157 - DOI - PMC - PubMed

