Blocking Mitotic Exit of Ovarian Cancer Cells by Pharmaceutical Inhibition of the Anaphase-Promoting Complex Reduces Chromosomal Instability
- PMID: 30851646
- PMCID: PMC6407080
- DOI: 10.1016/j.neo.2019.01.007
Blocking Mitotic Exit of Ovarian Cancer Cells by Pharmaceutical Inhibition of the Anaphase-Promoting Complex Reduces Chromosomal Instability
Abstract
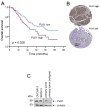
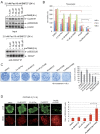
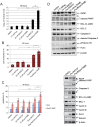
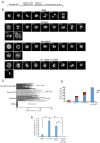
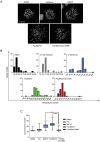
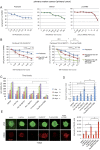
Paclitaxel is a frontline drug for the treatment of epithelial ovarian cancer (EOC). However, following paclitaxel-platinum based chemotherapy, tumor recurrence occurs in most ovarian cancer patients. Chromosomal instability (CIN) is a hallmark of cancer and represents genetic variation fueling tumor adaptation to cytotoxic effects of anticancer drugs. In this study, our Kaplan-Meier analysis including 263 ovarian cancer patients (stages I/II) revealed that high Polo-like kinase (PLK) 1 expression correlates with bad prognosis. To evaluate the role of PLK1 as potential cancer target within a combinatorial trial, we induced strong mitotic arrest in ovarian cancer cell lines by synergistically co-targeting microtubules (paclitaxel) and PLK1 (BI6727) followed by pharmaceutical inhibition of the Anaphase-Promoting Complex (APC/C) using proTAME. In short- and long-term experiments, this triple treatment strongly activated apoptosis in cell lines and primary ovarian cells derived from cancer patients. Mechanistically, BI6727/paclitaxel/proTAME stabilize Cyclin B1 and trigger mitotic arrest, which initiates mitochondrial apoptosis by inactivation of antiapoptotic BCL-2 family proteins, followed by activation of caspase-dependent effector pathways. This triple treatment prevented endoreduplication and reduced CIN, two mechanisms that are associated with aggressive tumors and the acquisition of drug resistance. This "two-punch strategy" (strong mitotic arrest followed by blocking mitotic exit) has important implications for developing paclitaxel-based combinatorial treatments in ovarian cancer.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17:65–74. - PubMed
-
- Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–239. - PubMed
-
- Bolis G, Scarfone G, Raspagliesi F, Mangili G, Danese S, Scollo P, Lo Russo D, Villa A, Aimone PD, Scambia G. Paclitaxel/carboplatin versus topotecan/paclitaxel/carboplatin in patients with FIGO suboptimally resected stage III-IV epithelial ovarian cancer a multicenter, randomized study. Eur J Cancer. 2010;46:2905–2912. - PubMed
-
- du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

