HSP60/10 chaperonin systems are inhibited by a variety of approved drugs, natural products, and known bioactive molecules
- PMID: 30852084
- PMCID: PMC6450568
- DOI: 10.1016/j.bmcl.2019.02.028
HSP60/10 chaperonin systems are inhibited by a variety of approved drugs, natural products, and known bioactive molecules
Abstract
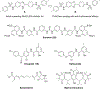
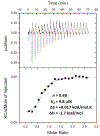
All living organisms contain a unique class of molecular chaperones called 60 kDa heat shock proteins (HSP60 - also known as GroEL in bacteria). While some organisms contain more than one HSP60 or GroEL isoform, at least one isoform has always proven to be essential. Because of this, we have been investigating targeting HSP60 and GroEL chaperonin systems as an antibiotic strategy. Our initial studies focused on applying this antibiotic strategy for treating African sleeping sickness (caused by Trypanosoma brucei parasites) and drug-resistant bacterial infections (in particular Methicillin-resistant Staphylococcus aureus - MRSA). Intriguingly, during our studies we found that three known antibiotics - suramin, closantel, and rafoxanide - were potent inhibitors of bacterial GroEL and human HSP60 chaperonin systems. These findings prompted us to explore what other approved drugs, natural products, and known bioactive molecules might also inhibit HSP60 and GroEL chaperonin systems. Initial high-throughput screening of 3680 approved drugs, natural products, and known bioactives identified 161 hit inhibitors of the Escherichia coli GroEL chaperonin system (4.3% hit rate). From a purchased subset of 60 hits, 29 compounds (48%) re-confirmed as selective GroEL inhibitors in our assays, all of which were nearly equipotent against human HSP60. These findings illuminate the notion that targeting chaperonin systems might be a more common occurrence than we previously appreciated. Future studies are needed to determine if the in vivo modes of action of these approved drugs, natural products, and known bioactive molecules are related to GroEL and HSP60 inhibition.
Keywords: Chaperonin; GroEL; GroES; HSP10; HSP60; Molecular chaperone; Natural products; Proteostasis; Small molecule inhibitors.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
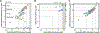
References
-
- Hartl FU; Bracher A; Hayer-Hartl M Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. - PubMed
-
- Stefani M; Dobson CM Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. (Berl.) 2003, 81, 678–699. - PubMed
-
- Bao YP; Cook LJ; O’Donovan D; Uyama E; Rubinsztein DC Mammalian, yeast, bacterial, and chemical chaperones reduce aggregate formation and death in a cell model of oculopharyngeal muscular dystrophy. J. Biol. Chem 2002, 277, 12263–12269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

