Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort
- PMID: 30852552
- PMCID: PMC6517861
- DOI: 10.1136/annrheumdis-2018-213455
Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort
Abstract
Objectives: To determine whether progressive skin fibrosis is associated with visceral organ progression and mortality during follow-up in patients with diffuse cutaneous systemic sclerosis (dcSSc).
Methods: We evaluated patients from the European Scleroderma Trials and Research database with dcSSc, baseline modified Rodnan skin score (mRSS) ≥7, valid mRSS at 12±3 months after baseline and ≥1 annual follow-up visit. Progressive skin fibrosis was defined as an increase in mRSS >5 and ≥25% from baseline to 12±3 months. Outcomes were pulmonary, cardiovascular and renal progression, and all-cause death. Associations between skin progression and outcomes were evaluated by Kaplan-Meier survival analysis and multivariable Cox regression.
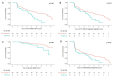
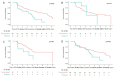
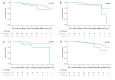
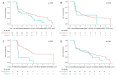
Results: Of 1021 included patients, 78 (7.6%) had progressive skin fibrosis (skin progressors). Median follow-up was 3.4 years. Survival analyses indicated that skin progressors had a significantly higher probability of FVC decline ≥10% (53.6% vs 34.4%; p<0.001) and all-cause death (15.4% vs 7.3%; p=0.003) than non-progressors. These significant associations were also found in subgroup analyses of patients with either low baseline mRSS (≤22/51) or short disease duration (≤15 months). In multivariable analyses, skin progression within 1 year was independently associated with FVC decline ≥10% (HR 1.79, 95% CI 1.20 to 2.65) and all-cause death (HR 2.58, 95% CI 1.31 to 5.09).
Conclusions: Progressive skin fibrosis within 1 year is associated with decline in lung function and worse survival in dcSSc during follow-up. These results confirm mRSS as a surrogate marker in dcSSc, which will be helpful for cohort enrichment in future trials and risk stratification in clinical practice.
Keywords: all-cause death; diffuse cutaneous systemic sclerosis; lung function decline; progressive skin fibrosis; visceral organ progression.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: OD has obtained research support from Bayer, Sanofi, Ergonex, Boehringer Ingelheim, Actelion and Pfizer. He is a scientific consultant for 4D Science, Actelion, Active Biotec, Bayer, BiogenIdec, BMS, Boehringer Ingelheim, ChemoAb, EpiPharm, Ergonex, espeRare foundation, Genentech/Roche, GSK, Inventiva, Lilly, Medac, MedImmune, Pharmacyclics, Pfizer, Serodapharm and Sinoxa, and has a patent licensed on mir-29 for the treatment of systemic sclerosis. DK has consultancy relationships and/or has received grant/research support from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech/Roche, NIH, Pfizer, Sanofi-Aventis Pharmaceuticals, Actelion Pharmaceuticals US, Chemomab, Corbus, Covis, Cytori, Eicos, EMD Serono, Gilead, GlaxoSmithKline and UCB Pharma. He is a shareholder of Eicos. CPD has consultancy relationships with and/or has received speakers’ bureau fees from Actelion Pharmaceuticals US, Bayer AG, GlaxoSmithKline, CSL Behring, Merck-Serono, Roche Pharmaceuticals, Genentech and Biogen IDEC Inc., Inventiva, Sanofi-Aventis Pharmaceuticals and Boehringer Ingelheim. JEP has consultancy relationships with and/or has received grant/research support from Actelion, Bayer AG, Bristol-Myers Squibb, Merck, Pfizer Inc. and Roche. MM-C has consultancy relationships and/or has received grant/research support from Pfizer, Bristol-Myers Squibb, Actelion, UCB Pharma, Bayer, ChemomAb, Genentech/Roche, Inventiva and Lilly. YA has consultancy relationships with and/or has received grant/research support from Actelion, Pharmaceuticals US, Bayer AG, Bristol-Myers Squibb, Inventiva, Medac, Pfizer Inc., Roche Pharmaceuticals, Genentech and Biogen IDEC Inc., Sanofi-Aventis Pharmaceuticals and Servier. JdOP and JC are employees of Bayer. WW, SJ and NG have nothing to disclose.
Figures
References
-
- Khanna D, Distler JHW, Sandner P, et al. Emerging strategies for treatment of systemic sclerosis. J Scleroderma Relat Disord 2016;1:186–93. 10.5301/jsrd.5000207 - DOI
-
- Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol 1993;20:1892–6. - PubMed

