Magnesium Flux Modulates Ribosomes to Increase Bacterial Survival
- PMID: 30853217
- PMCID: PMC6814349
- DOI: 10.1016/j.cell.2019.01.042
Magnesium Flux Modulates Ribosomes to Increase Bacterial Survival
Abstract
Bacteria exhibit cell-to-cell variability in their resilience to stress, for example, following antibiotic exposure. Higher resilience is typically ascribed to "dormant" non-growing cellular states. Here, by measuring membrane potential dynamics of Bacillus subtilis cells, we show that actively growing bacteria can cope with ribosome-targeting antibiotics through an alternative mechanism based on ion flux modulation. Specifically, we observed two types of cellular behavior: growth-defective cells exhibited a mathematically predicted transient increase in membrane potential (hyperpolarization), followed by cell death, whereas growing cells lacked hyperpolarization events and showed elevated survival. Using structural perturbations of the ribosome and proteomic analysis, we uncovered that stress resilience arises from magnesium influx, which prevents hyperpolarization. Thus, ion flux modulation provides a distinct mechanism to cope with ribosomal stress. These results suggest new approaches to increase the effectiveness of ribosome-targeting antibiotics and reveal an intriguing connection between ribosomes and the membrane potential, two fundamental properties of cells.
Keywords: antibiotics; bacterial survival; cations; ion flux; ion transporters; magnesium; membrane potential; ribosomes; single-cell dynamics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures

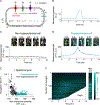


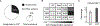

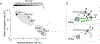
Comment in
-
Unlocking new potentials.Nat Rev Microbiol. 2019 Jun;17(6):334-335. doi: 10.1038/s41579-019-0190-9. Nat Rev Microbiol. 2019. PMID: 30911143 No abstract available.
References
-
- Akanuma G, Nanamiya H, Natori Y, Yano K, Suzuki S, Omata S, Ishizuka M, Sekine Y, and Kawamura F (2012). Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. J. Bacteriol 194, 6282–6291. - PMC - PubMed
-
- Arganda-Carreras I, Kaynig V, Rueden C, Eliceiri KW, Schindelin J, Cardona A, and Seung HS (2017). Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 33, 2424–2426. - PubMed
-
- Balaban NQ, Merrin J, Chait R, Kowalik L, and Leibler S (2004). Bacterial persistence as a phenotypic switch. Science 305, 1622–1625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

