Point of care testing for infectious diseases
- PMID: 30853460
- PMCID: PMC6462423
- DOI: 10.1016/j.cca.2019.03.008
Point of care testing for infectious diseases
Abstract
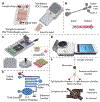
Infectious diseases are caused by pathogenic microorganisms and can be transmitted between individuals and populations thus threatening the general public health and potentially the economy. Efficient diagnostic tools are needed to provide accurate and timely guidance for case identification, transmission disruption and appropriate treatment administration. Point of care (POC) tests provide actionable results near the patient and thereby serve as a personal "radar". In this review, we review clinical needs for POC testing for several major pathogens, including malaria parasites, human immunodeficiency virus (HIV), human papillomavirus (HPV), dengue, Ebola and Zika viruses and Mycobacterium tuberculosis (TB). We compare different molecular approaches, including pathogen nucleic acid and protein, circulating microRNA and antibodies, used in the POC tests. Finally, we review recent advances in novel POC technologies focusing on microfluidic and plasmonic-based approaches.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
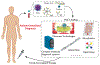


References
-
- Garcia-Basteiro AL, DiNardo A, Saavedra B, Silva DR, Palmero D, Gegia M, Migliori GB, Duarte R, Mambuque E, Centis R, Cuevas LE, Izco S, Theron G, Point of care diagnostics for tuberculosis, Pulmonology 24(2) (2018) 73–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

