Gene Expression Profile of Olfactory Transduction Signaling in an Animal Model of Human Multiple Sclerosis
- PMID: 30853826
- PMCID: PMC6401553
- DOI: 10.5607/en.2019.28.1.74
Gene Expression Profile of Olfactory Transduction Signaling in an Animal Model of Human Multiple Sclerosis
Abstract
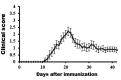
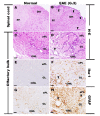
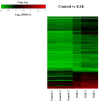
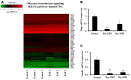
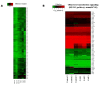
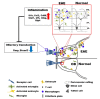
Olfactory dysfunction occurs in multiple sclerosis in humans, as well as in an animal model of experimental autoimmune encephalomyelitis (EAE). The aim of this study was to analyze differentially expressed genes (DEGs) in olfactory bulb of EAE-affected mice by next generation sequencing, with a particular focus on changes in olfaction-related signals. EAE was induced in C57BL/6 mice following immunization with myelin oligodendrocyte glycoprotein and adjuvant. Inflammatory lesions were identified in the olfactory bulbs as well as in the spinal cord of immunized mice. Analysis of DEGs in the olfactory bulb of EAE-affected mice revealed that 44 genes were upregulated (and which were primarily related to inflammatory mediators), while 519 genes were downregulated; among the latter, olfactory marker protein and stomatin-like 3, which have been linked to olfactory signal transduction, were significantly downregulated (log2 [fold change] >1 and p-value <0.05). These findings suggest that inflammation in the olfactory bulb of EAE-affected mice is associated with the downregulation of some olfactory signal transduction genes, particularly olfactory marker protein and stomatin-like 3, which may lead to olfactory dysfunction in an animal model of human multiple sclerosis.
Keywords: Differentially expressed gene; Experimental autoimmune encephalomyelitis; Olfactory bulb; Olfactory marker protein.
Figures

Similar articles
-
Retinal transcriptome profiling identifies novel candidate genes associated with visual impairment in a mouse model of multiple sclerosis.Anim Cells Syst (Seoul). 2023 Oct 4;27(1):219-233. doi: 10.1080/19768354.2023.2264354. eCollection 2023. Anim Cells Syst (Seoul). 2023. PMID: 37808551 Free PMC article.
-
Olfactory Dysfunction in Autoimmune Central Nervous System Neuroinflammation.Mol Neurobiol. 2018 Nov;55(11):8499-8508. doi: 10.1007/s12035-018-1001-4. Epub 2018 Mar 20. Mol Neurobiol. 2018. PMID: 29557516
-
Key Genes in Olfactory Disorder in Experimental Autoimmune Encephalomyelitis Identified by Transcriptomic Analysis of the Olfactory Bulbs.Mol Neurobiol. 2024 Aug;61(8):5771-5786. doi: 10.1007/s12035-024-03923-0. Epub 2024 Jan 17. Mol Neurobiol. 2024. PMID: 38233686
-
Upregulation of Cathepsins in Olfactory Bulbs Is Associated with Transient Olfactory Dysfunction in Mice with Experimental Autoimmune Encephalomyelitis.Mol Neurobiol. 2020 Aug;57(8):3412-3423. doi: 10.1007/s12035-020-01952-z. Epub 2020 Jun 11. Mol Neurobiol. 2020. PMID: 32529488
-
Active Induction of Experimental Autoimmune Encephalomyelitis (EAE) with MOG35-55 in the Mouse.Methods Mol Biol. 2018;1791:227-232. doi: 10.1007/978-1-4939-7862-5_17. Methods Mol Biol. 2018. PMID: 30006713
Cited by
-
Changes in structural plasticity of hippocampal neurons in an animal model of multiple sclerosis.Zool Res. 2024 Mar 18;45(2):398-414. doi: 10.24272/j.issn.2095-8137.2023.309. Zool Res. 2024. PMID: 38485508 Free PMC article.
-
Neuroinflammation causes mitral cell dysfunction and olfactory impairment in a multiple sclerosis model.J Neuroinflammation. 2025 Mar 8;22(1):71. doi: 10.1186/s12974-025-03388-5. J Neuroinflammation. 2025. PMID: 40057769 Free PMC article.
-
Soluble Epoxide Hydrolase Inhibitor Ameliorates Olfactory Dysfunction, Modulates Microglia Polarization, and Attenuates Neuroinflammation after Ischemic Brain Injury.J Neuroimmune Pharmacol. 2024 Oct 17;19(1):54. doi: 10.1007/s11481-024-10155-5. J Neuroimmune Pharmacol. 2024. PMID: 39417923
-
Retinal transcriptome profiling identifies novel candidate genes associated with visual impairment in a mouse model of multiple sclerosis.Anim Cells Syst (Seoul). 2023 Oct 4;27(1):219-233. doi: 10.1080/19768354.2023.2264354. eCollection 2023. Anim Cells Syst (Seoul). 2023. PMID: 37808551 Free PMC article.
-
Effects of In Vivo Gluten Challenge on PBMC Gene Expression Profiles in Diet Treated Celiac Disease.Front Immunol. 2020 Dec 11;11:594243. doi: 10.3389/fimmu.2020.594243. eCollection 2020. Front Immunol. 2020. PMID: 33362776 Free PMC article.

