Omi/HtrA2 Protease Associated Cell Apoptosis Participates in Blood-Brain Barrier Dysfunction
- PMID: 30853894
- PMCID: PMC6395387
- DOI: 10.3389/fnmol.2019.00048
Omi/HtrA2 Protease Associated Cell Apoptosis Participates in Blood-Brain Barrier Dysfunction
Abstract
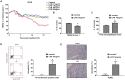
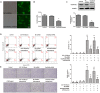
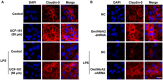
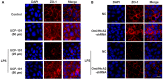
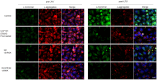
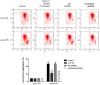
Background: Omi/HtrA2 is a proapoptotic mitochondrial serine protease involved in caspase-dependent cell apoptosis, translocating from mitochondria to the cytosol after an apoptotic insult. Our previous study indicated pre-treatment with UCF-101, a specific inhibitor of Omi/HtrA2, could significantly reduce neuronal apoptosis and attenuate sepsis-induced cognitive dysfunction. Various hypotheses involving blood-brain-barrier (BBB) disruption have been proposed to account for sepsis-associated encephalopathy (SAE). Here, we attempted to explore whether interference of Omi/HtrA2 by RNA interference or UCF-101 pre-treatment can improve sepsis-induced disruption of BBB using human cerebral microvascular endothelial cell line (hCMEC/D3) in vitro and if so, to explore mechanisms involved Omi/HtrA2 protease mediates BBB disruption in SAE. Methods: hCMEC/D3 cell monolayers were intervened by different concentrations of LPS (0-50 μg/mL) over experimental period. Pharmacological or gene interventions (by silencing RNA of Omi/HtrA2) were used to study molecular mechanisms involved in sepsis-associated Omi/HtrA2 translocation, cell apoptosis and BBB dysfunction. BBB function was assessed by trans-endothelial electrical resistance (TEER) and permeability to labeled dextrans (FITC-4kDa). Tight junction (TJ) integrity was assessed by immunofluorescence, western blotting and transmission electron microscopic (TEM) analyses. Apoptosis was determined using flow cytometry and TUNEL assay. Mitochondrial membrane potential (MMP) and oxidative stress were also investigated. Results: LPS affects hCMEC/D3 TJ permeability in a concentration- and time-dependent manner. LPS intervention resulted in a significant disruption of BBB, as manifested by decreased TEER (by ~26%) and a parallel increased paracellular permeability to FITC- (4kDa) dextrans through hCMEC/D3 monolayers. The inhibition of Omi/HtrA2 by UCF-101 or Omi/HtrA2 shRNA reduced LPS-induced brain endothelial cell apoptosis, and resulted in significant improvement on LPS-induced BBB disruption as well as decreased occludin, claudin-5 and ZO-1 expressions. Omi/HtrA2 manipulated endothelial cell apoptosis by shifting into cytosol and inducing X-linked inhibitor of apoptosis protein (XIAP) degradation. UCF-101 administration or Omi/HtrA2 shRNA intervention did attenuate the degradation of XIAP, Poly ADP-ribose polymerase (PARP) cleavage, and caspase-3 cleavage. However, only UCF-101 partly prevented the mobilization of Omi/HtrA2 from the mitochondria to the cytosol after LPS intervention. That abrogation of Omi/HtrA2 by UCF-101 or Omi/HtrA2 shRNA resulted in a significant improvement on LPS-induced decrease of MMP. Oxidative stress was significantly increased in the LPS treated group compared to the control or NC-shRNA group. However, abrogation of Omi/HtrA2 by UCF-101 or Omi/HtrA2 shRNA did not significantly improve oxidative injury. Conclusions: Our study indicated an important role of Omi/HtrA2 in manipulating LPS-induced cell apoptosis and BBB integrity by translocating from mitochondria into cytosol in brain endothelial cells. Omi/HtrA2 induced mitochondrial pathway apoptosis, which involves inhibition of an important antiapoptotic protein XIAP and influence on MMP. Therapeutic methods that inhibit Omi/HtrA2 function may provide a novel therapeutic measure to septic encephalopathy.
Keywords: Omi/HtrA2; UCF-101; blood-brain barrier; hCMEC/D3 cells; sepsis.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

