Metformin Suppresses Hypopharyngeal Cancer Growth by Epigenetically Silencing Long Non-coding RNA SNHG7 in FaDu Cells
- PMID: 30853913
- PMCID: PMC6395377
- DOI: 10.3389/fphar.2019.00143
Metformin Suppresses Hypopharyngeal Cancer Growth by Epigenetically Silencing Long Non-coding RNA SNHG7 in FaDu Cells
Abstract
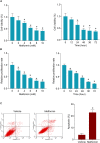
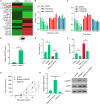
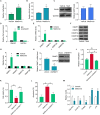
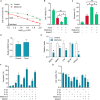
Local recurrence after therapy remains a challenging problem for hypopharyngeal cancer (HPC) due to the chemotherapy resistance. Metformin is associated with reduced cancer risk through promoting global DNA methylation in cancer cells by controlling S-adenosylhomocysteine (SAHH) activity. However, the mechanisms by which metformin inhibits HPC remain elusive. In this study, we aim to investigate the role of metformin in HPC and illustrate the mechanism by which metformin regulates long non-coding RNAs (lncRNAs) expression. CCK-8 and annexin-V/PI double staining were performed to analyze the cell viability and apoptosis. LncRNA microarray analysis, QPCR, methylation specific PCR, Western blot and RNA Immunoprecipitation were performed to analyze the molecular mechanism, Here, we report that metformin inhibits FaDu cell proliferation in time- and dose-dependent manner by suppressing lncRNA SNHG7. Further investigations revealed that SNHG7 interacted with SAHH and metformin decreased SNHG7 expression by activating SAHH activity. Increased SAHH activity resulted in upregulating DNMT1 expression, leading to hypermethylation of SNHG7 promotor. In addition, upregulation of SNHG7 was associated with advanced stage. The patients with high SNHG7 have lower overall survival than that of with low SNHG7. Interestingly, SNHG7 levels were higher in taxol resistant patients than in taxol sensitive patients. Metformin sensitizes FaDu cells to taxol and irradiation through decreasing SNHG7. In conclusion, our recent study demonstrates that metformin inhibits FaDu cell proliferation by decreasing SNHG7 expression via SAHH-mediated DNA methylation. These findings indicate that combined metformin with paclitaxel or irradiation would be a novel therapeutic strategy to overcome resistance and prevent recurrence in HPC.
Keywords: DNA methylation; SNHG7; hypopharyngeal cancer; long non-coding RNAs; metformin.
Figures

References
-
- Adeberg S., Bernhardt D., Harrabi S. B., Nicolay N. H., Horner-Rieber J., Konig L., et al. (2017). Metformin enhanced in vitro radiosensitivity associates with G2/M cell cycle arrest and elevated adenosine-5’-monophosphate-activated protein kinase levels in glioblastoma. Radiol. Oncol. 51 431–437. 10.1515/raon-2017-0042 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

