Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer
- PMID: 30853990
- PMCID: PMC6378048
- DOI: 10.1155/2019/5268342
Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer
Abstract
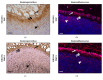
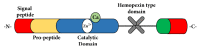
Several studies have shown that the dentin-resin interface is unstable due to poor infiltration of resin monomers into the demineralized dentin matrix. This phenomenon is related to the incomplete infiltration of the adhesive system into the network of exposed collagen fibrils, mainly due to the difficulty of displacement and subsequent replacement of trapped water between interfibrillar spaces, avoiding adequate hybridization within the network of collagen fibrils. Thus, unprotected fibrils are exposed to undergo denaturation and are susceptible to cyclic fatigue rupture after being subjected to repetitive loads during function. The aqueous inclusions within the hybrid layer serve as a functional medium for the hydrolysis of the resin matrix, giving rise to the activity of esterases and collagenolytic enzymes, such as matrix metalloproteinases, which play a fundamental role in the degradation process of the hybrid layer. Achieving better interdiffusion of the adhesive system in the network of collagen fibrils and the substrate stability in the hybrid layer through different strategies are key events for the interfacial microstructure to adequately function. Hence, it is important to review the factors related to the mechanisms of degradation and stabilization of the hybrid layer to support the implementation of new materials and techniques in the future. The enzymatic degradation of collagen matrix, together with resin leaching, has led to seeking strategies that inhibit the endogenous proteases, cross-linking the denudated collagen fibrils and improving the adhesive penetration removing water from the interface. Some of dentin treatments have yielded promising results and require more research to be validated. A longer durability of adhesive restorations could resolve a variety of clinical problems, such as microleakage, recurrent caries, postoperative sensitivity, and restoration integrity.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

