Understanding the burden of refractory myasthenia gravis
- PMID: 30854027
- PMCID: PMC6399761
- DOI: 10.1177/1756286419832242
Understanding the burden of refractory myasthenia gravis
Abstract
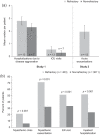
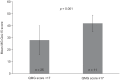
Myasthenia gravis (MG) is an autoantibody-mediated disease that compromises the acetylcholine receptors or associated structures of the postsynaptic membrane of the neuromuscular junction. This leads to impaired neuromuscular transmission and subsequent fluctuating fatigability and weakness of ocular, bulbar, and limb skeletal muscles. Over the past few decades, there have been significant advances in our understanding of the disease pathophysiology and improvements in prognosis due to intensive care medicine and immunomodulation. Despite this, an estimated 10-20% of patients with MG do not achieve an adequate response, are intolerant to conventional treatment, or require chronic treatment with intravenous immunoglobulins or plasma separation procedures. Such patients are regarded as having MG that is 'refractory' to treatment and may represent a distinct clinical subgroup. Because the majority of patients with MG have well-controlled disease, the burden of illness in the minority with refractory disease is poorly understood and may be underestimated. However, clinically these patients are liable to experience extreme fatigue, considerable disability owing to uncontrolled symptoms, and frequent myasthenic crises and hospitalizations. Both acute adverse effects and an increased risk of comorbidity from treatment regimens may contribute to reduced quality of life. As yet, little is known concerning the impact of refractory MG on mental health and health-related quality of life. This review aims to highlight the burden of disease and unmet needs in patients with refractory MG.
Keywords: burden; definition; disability; quality of life; refractory myasthenia gravis; side effects; tolerability; treatment; unmet need.
Conflict of interest statement
Conflict of interest statement: Dr Schneider-Gold, Dr Hagenacker, and Dr Melzer have received speaker’s honoraria and honoraria for attendance at advisory boards from Alexion Pharmaceuticals. Dr Ruck has received speaker’s honoraria from Alexion Pharmaceuticals.
Figures
References
-
- Verschuuren JJ, Huijbers MG, Plomp JJ, et al. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun Rev 2013; 12: 918–923. - PubMed
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14: 1023–1036. - PubMed
-
- Drachman DB, Kaminski HJ. Neuromuscular junction as Achilles’ heel: yet another autoantibody? Neurology 2014; 82: 1942–1943. - PubMed
-
- Gasperi C, Melms A, Schoser B, et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology 2014; 82: 1976–1983. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

