Effects of probiotics on chemotherapy in patients with lung cancer
- PMID: 30854059
- PMCID: PMC6365978
- DOI: 10.3892/ol.2019.9906
Effects of probiotics on chemotherapy in patients with lung cancer
Abstract
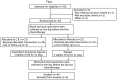
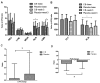
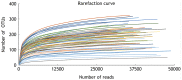
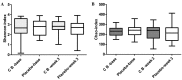
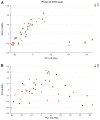
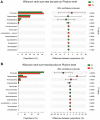
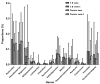
Chemotherapy damages the intestinal mucosa, causing adverse gastrointestinal reactions. Clostridium butyricum (C. butyricum) reduces the incidence of diarrhea in digestive diseases, including inflammatory bowel disease. Therefore, the aim of the present study was to investigate the role of C. butyricum in patients undergoing chemotherapy. A total of 41 participants with lung cancer were enrolled, and divided into the C. butyricum (CB) or placebo group using 1:1 randomization to obtain 20 CB and 21 placebo participants. On the first and last day of the 3-week intervention, blood and stool samples were collected and analyzed. To analyze stool flora, 16S ribosomal RNA sequencing was performed. The incidence of chemotherapy-induced diarrhea was lower in the CB group compared with the placebo group. The lymphocyte count and platelet/lymphocyte ratio (PLR) was markedly altered between the two groups. Neutrophil/lymphocyte ratio (NLR) and PLR decreased within the CB group. At week 3, the lymphocyte/monocyte ratio (LMR) was higher in the CB group compared with the placebo group. Alterations in lymphocyte subsets and immunoglobulin levels were not significantly different. Albumin (ALB) level and weight did not differ significantly between the two groups. At 3 weeks the total flora diversity did not decrease in either group. Phyla in the CB group varied slightly, while the proportion of Firmicutes in the placebo group decreased significantly. No statistically significant difference was observed between the two groups, though the genera producing short-chain fatty acids tended to increase, and the pathogenic genera tended to decrease in the CB group, which was almost the opposite of the observation in the placebo group. Operational taxonomy unit analysis revealed a notable increase in beneficial flora, including the Clostridium and Lactobacillus genera of the CB group, compared with the placebo group. The present study highlighted that C. butyricum reduced chemotherapy-induced diarrhea in patients with lung cancer, reduced the systemic inflammatory response system and encouraged homeostatic maintenance.
Keywords: chemotherapy; intestinal microflora; probiotic; systemic inflammatory response.
Figures
References
-
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
