Needs management in families affected by childhood-onset dystrophinopathies
- PMID: 30854202
- PMCID: PMC6399767
- DOI: 10.1177/2050312119834470
Needs management in families affected by childhood-onset dystrophinopathies
Abstract
Purpose: To collect information about the needs of families affected by childhood-onset dystrophinopathies residing in the United States.
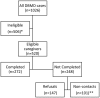
Methods: Individuals with an eligible dystrophinopathy were identified by the Muscular Dystrophy Surveillance, Tracking, and Research network. Between September 2008 and December 2012, 272 caregivers completed a 48-item survey about needs related to information, healthcare services, psychosocial issues, finances, caregiver demographics, and the individual's functioning.
Results: Overall, at least 80% of the survey items were identified as needs for more than one-half of caregivers. Among the needs identified, physical health and access to information were currently managed for most caregivers. Items identified as needed but managed less consistently were funding for needs not covered by insurance and psychosocial support.
Conclusions: Healthcare providers, public health practitioners, and policymakers should be aware of the many needs reported by caregivers, and focus on addressing gaps in provision of needed financial and psychosocial services.
Keywords: Becker muscular dystrophy; Duchenne muscular dystrophy; caregivers; dystrophinopathy; needs assessment; survey.
Conflict of interest statement
Declaration of conflicting interests: K.D.M. receives funding from National Institutes of Health (NIH) grant 2 U54 NS053672-11, the Friedreich’s Ataxia Research Alliance, the Centers for Disease Control and Prevention (DD000189), PTC Therapeutics Inc, Sarepta Therapeutics Inc, Pfizer Inc, FibroGen Inc, AMO, BMS, Santhera, Acceleron, Takeda, Reata, and Intalfarmaco. Previously, funding was received from GlaxoSmithKline, Eli Lilly and Company, Prosensa Therapeutics BV/BioMarin Pharmaceutical Inc, ViroPharma Inc, Marathon Pharmaceuticals LLC, aTyr Pharma Inc, and Horizon Pharma Ireland Ltd. K.D.M. is an advisory board member for Sarepta Therapeutics Inc and Santhera. K.D.M. is a scientific board member for the Muscular Dystrophy Association and the FSH Society. K.D.M. received no personal funding aside from travel expense reimbursement. The remaining authors (K.M.C., K.E., C.T., P.A.R., and S.K.P.) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Emery AE. The muscular dystrophies. Lancet 2002; 359(9307): 687–695. - PubMed
-
- Balaban B, Matthews DJ, Clayton GH, et al. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil 2005; 84(11): 843–850. - PubMed
-
- Barber BJ, Andrews JG, Lu Z, et al. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy. J Pediatr 2013; 163(4): 1080–1084.e1. - PubMed
-
- Biggar WD, Harris VA, Eliasoph L, et al. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord 2006; 16(4): 249–255. - PubMed
-
- Houde S, Filiatrault M, Fournier A, et al. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediatr Neurol 2008; 38(3): 200–206. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

