Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments
- PMID: 30854368
- PMCID: PMC6396078
- DOI: 10.3389/fchem.2019.00048
Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments
Abstract
Near-infrared (12,500-4,000 cm-1; 800-2,500 nm) spectroscopy is the hallmark for one of the most rapidly advancing analytical techniques over the last few decades. Although it is mainly recognized as an analytical tool, near-infrared spectroscopy has also contributed significantly to physical chemistry, e.g., by delivering invaluable data on the anharmonic nature of molecular vibrations or peculiarities of intermolecular interactions. In all these contexts, a major barrier in the form of an intrinsic complexity of near-infrared spectra has been encountered. A large number of overlapping vibrational contributions influenced by anharmonic effects create complex patterns of spectral dependencies, in many cases hindering our comprehension of near-infrared spectra. Quantum mechanical calculations commonly serve as a major support to infrared and Raman studies; conversely, near-infrared spectroscopy has long been hindered in this regard due to practical limitations. Advances in anharmonic theories in hyphenation with ever-growing computer technology have enabled feasible theoretical near-infrared spectroscopy in recent times. Accordingly, a growing number of quantum mechanical investigations aimed at near-infrared region has been witnessed. The present review article summarizes these most recent accomplishments in the emerging field. Applications of generalized approaches, such as vibrational self-consistent field and vibrational second order perturbation theories as well as their derivatives, and dense grid-based studies of vibrational potential, are overviewed. Basic and applied studies are discussed, with special attention paid to the ones which aim at improving analytical spectroscopy. A remarkable potential arises from the growing applicability of anharmonic computations to solving the problems which arise in both basic and analytical near-infrared spectroscopy. This review highlights an increased value of quantum mechanical calculations to near-infrared spectroscopy in relation to other kinds of vibrational spectroscopy.
Keywords: NIRS; anharmonic methods; near-infrared; spectra simulation; theoretical spectroscopy.
Figures





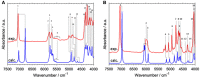
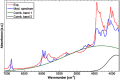
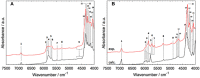

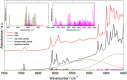
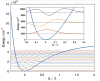

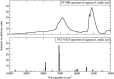

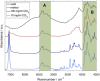
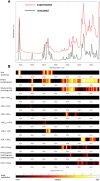
References
-
- Ahmad M. (ed.). (2017). Fatty Acids. Oxford: Academic Press; AOCS Press.
-
- Alcalà M., Blanco M., Moyano D., Broad N. W., O'Brien N., Friedrich D., et al. (2013). Qualitative and quantitative pharmaceutical analysis with a novel handheld miniature near-infrared spectrometer. J. Near Infrared Spectrosc. 21, 445–447. 10.1255/jnirs.1084 - DOI
-
- Altinpinarn S., Sorak D., Siesler H. W. (2013). Near infrared spectroscopic analysis of hydrocarbon contaminants in soil with a hand-held spectrometer. J. Near Infrared Spectrosc. 21, 511–521. 10.1255/jnirs.1079 - DOI
Publication types
LinkOut - more resources
Full Text Sources

