One-Year Treatment with Olanzapine Depot in Female Rats: Metabolic Effects
- PMID: 30854556
- PMCID: PMC6499254
- DOI: 10.1093/ijnp/pyz012
One-Year Treatment with Olanzapine Depot in Female Rats: Metabolic Effects
Abstract
Background: Antipsychotic drugs can negatively affect the metabolic status of patients, with olanzapine as one of the most potent drugs. While patients are often medicated for long time periods, experiments in rats typically run for 1 to 12 weeks, showing olanzapine-related weight gain and increased plasma lipid levels, with transcriptional upregulation of lipogenic genes in liver and adipose tissue. It remains unknown whether metabolic status will deteriorate with time.
Methods: To examine long-term metabolic effects, we administered intramuscular long-acting injections of olanzapine (100 mg/kg BW) or control substance to female rats for up to 13 months.
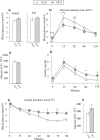
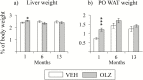
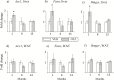
Results: Exposure to olanzapine long-acting injections led to rapid weight gain, which was sustained throughout the experiment. At 1, 6, and 13 months, plasma lipid levels were measured in separate cohorts of rats, displaying no increase. Hepatic transcription of lipid-related genes was transiently upregulated at 1 month. Glucose and insulin tolerance tests indicated insulin resistance in olanzapine-treated rats after 12 months.
Conclusion: Our data show that the continuous increase in body weight in response to long-term olanzapine exposure was accompanied by surprisingly few concomitant changes in plasma lipids and lipogenic gene expression, suggesting that adaptive mechanisms are involved to reduce long-term metabolic adverse effects of this antipsychotic agent in rats.
Keywords: diabetes; long-term; olanzapine; rat; weight gain.
© The Author(s) 2019. Published by Oxford University Press on behalf of CINP.
Figures



References
-
- Aleixandre de Artinano A, Miguel Castro M (2009) Experimental rat models to study the metabolic syndrome. Br J Nutr 102:1246–1253. - PubMed
-
- Aravagiri M, Teper Y, Marder SR (1999) Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos 20:369–377. - PubMed
-
- Benarroch L, Kowalchuk C, Wilson V, Teo C, Guenette M, Chintoh A, Nesarajah Y, Taylor V, Selby P, Fletcher P, Remington GJ, Hahn MK (2016) Atypical antipsychotics and effects on feeding: from mice to men. Psychopharmacology (Berl) 233:2629–2653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

