GSK3β-SCFFBXW7α mediated phosphorylation and ubiquitination of IRF1 are required for its transcription-dependent turnover
- PMID: 30854564
- PMCID: PMC6511875
- DOI: 10.1093/nar/gkz163
GSK3β-SCFFBXW7α mediated phosphorylation and ubiquitination of IRF1 are required for its transcription-dependent turnover
Abstract
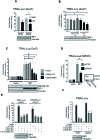
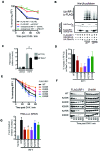
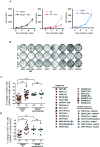
IRF1 (Interferon Regulatory Factor-1) is the prototype of the IRF family of DNA binding transcription factors. IRF1 protein expression is regulated by transient up-regulation in response to external stimuli followed by rapid degradation via the ubiquitin-proteasome system. Here we report that DNA bound IRF1 turnover is promoted by GSK3β (Glycogen Synthase Kinase 3β) via phosphorylation of the T181 residue which generates a phosphodegron for the SCF (Skp-Cul-Fbox) ubiquitin E3-ligase receptor protein Fbxw7α (F-box/WD40 7). This regulated turnover is essential for IRF1 activity, as mutation of T181 results in an improperly stabilized protein that accumulates at target promoters but fails to induce RNA-Pol-II elongation and subsequent transcription of target genes. Consequently, the anti-proliferative activity of IRF1 is lost in cell lines expressing T181A mutant. Further, cell lines with dysfunctional Fbxw7 are less sensitive to IRF1 overexpression, suggesting an important co-activator function for this ligase complex. As T181 phosphorylation requires both DNA binding and RNA-Pol-II elongation, we propose that this event acts to clear 'spent' molecules of IRF1 from transcriptionally engaged target promoters.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures








References
-
- Tanaka N., Ishihara M., Lamphier M.S., Nozawa H., Matsuyama T., Mak T.W., Aizawa S., Tokino T., Oren M., Taniguchi T.. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature. 1996; 382:816–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

