Aged-senescent cells contribute to impaired heart regeneration
- PMID: 30854802
- PMCID: PMC6516154
- DOI: 10.1111/acel.12931
Aged-senescent cells contribute to impaired heart regeneration
Abstract
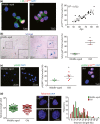
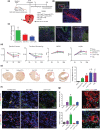
Aging leads to increased cellular senescence and is associated with decreased potency of tissue-specific stem/progenitor cells. Here, we have done an extensive analysis of cardiac progenitor cells (CPCs) isolated from human subjects with cardiovascular disease, aged 32-86 years. In aged subjects (>70 years old), over half of CPCs are senescent (p16INK4A , SA-β-gal, DNA damage γH2AX, telomere length, senescence-associated secretory phenotype [SASP]), unable to replicate, differentiate, regenerate or restore cardiac function following transplantation into the infarcted heart. SASP factors secreted by senescent CPCs renders otherwise healthy CPCs to senescence. Elimination of senescent CPCs using senolytics abrogates the SASP and its debilitative effect in vitro. Global elimination of senescent cells in aged mice (INK-ATTAC or wild-type mice treated with D + Q senolytics) in vivo activates resident CPCs and increased the number of small Ki67-, EdU-positive cardiomyocytes. Therapeutic approaches that eliminate senescent cells may alleviate cardiac deterioration with aging and restore the regenerative capacity of the heart.
Keywords: aging; cardiac regeneration; cardiac repair; myocardial infarction; p16INK4a; progenitor cells; senescence; senescence-associated secretory phenotype; senolytics.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures




References
-
- Canela, A. , Vera, E. , Klatt, P. , & Blasco, M. A. (2007). High‐throughput telomere length quantification by FISH and its application to human population studies. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5300–5305. 10.1073/pnas.0609367104 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

