Tβ4-Ac-SDKP pathway: Any relevance for the cardiovascular system?
- PMID: 30854877
- PMCID: PMC6824425
- DOI: 10.1139/cjpp-2018-0570
Tβ4-Ac-SDKP pathway: Any relevance for the cardiovascular system?
Abstract
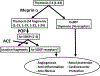
The last 20 years witnessed the emergence of the thymosin β4 (Tβ4)-N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) pathway as a new source of future therapeutic tools to treat cardiovascular and renal diseases. In this review article, we attempted to shed light on the numerous experimental findings pertaining to the many promising cardiovascular therapeutic avenues for Tβ4 and (or) its N-terminal derivative, Ac-SDKP. Specifically, Ac-SDKP is endogenously produced from the 43-amino acid Tβ4 by 2 successive enzymes, meprin α and prolyl oligopeptidase. We also discussed the possible mechanisms involved in the Tβ4-Ac-SDKP-associated cardiovascular biological effects. In infarcted myocardium, Tβ4 and Ac-SDKP facilitate cardiac repair after infarction by promoting endothelial cell migration and myocyte survival. Additionally, Tβ4 and Ac-SDKP have antifibrotic and anti-inflammatory properties in the arteries, heart, lungs, and kidneys, and stimulate both in vitro and in vivo angiogenesis. The effects of Tβ4 can be mediated directly through a putative receptor (Ku80) or via its enzymatically released N-terminal derivative Ac-SDKP. Despite the localization and characterization of Ac-SDKP binding sites in myocardium, more studies are needed to fully identify and clone Ac-SDKP receptors. It remains promising that Ac-SDKP or its degradation-resistant analogs could serve as new therapeutic tools to treat cardiac, vascular, and renal injury and dysfunction to be used alone or in combination with the already established pharmacotherapy for cardiovascular diseases.
Au cours des 20 dernières années, nous avons assisté à l’émergence de la voie de signalisation de la thymosine β4 (Tβ4)– N-acétyl-séryl-aspartyl-lysyl-proline (Ac-SDKP) comme nouvelle source d’outils thérapeutiques futurs pour le traitement de maladies cardiovasculaires et rénales. Dans cet article de synthèse, nous avons tenté de mettre en lumière les nombreux résultats expérimentaux quant aux nombreuses avenues thérapeutiques cardiovasculaires prometteuses pour le Tβ4 ou l’Ac-SDKP, son dérivé N-terminal. Spécifiquement, l’Ac-SDKP est un produit endogène obtenu à partir de Tβ4 de 43 acides aminés par 2 enzymes successives : la méprine et la prolyl oligopeptidase. Nous avons aussi discuté d’éventuels modes d’action pouvant jouer un rôle dans les effets biologiques cardiovasculaires associés au Tβ4–Ac-SDKP. Dans le myocarde infarci, le Tβ4 et l’Ac-SDKP facilitent la réparation du cœur après l’infarctus en favorisant la migration des cellules endothéliales et la survie des myocytes. En outre, le Tβ4 et l’Ac-SDKP ont des propriétés anti-fibrotiques et anti-inflammatoires dans les artères, le cœur, les poumons et les reins, et stimulent l’angiogenèse tant in vitro qu’in vivo. Les effets du Tβ4 peuvent être médiés directement par l’intermédiaire d’un récepteur putatif (Ku80) ou de l’Ac-SDKP, son dérivé N-terminal, libéré de manière enzymatique. En dépit de la localisation et de la caractérisation des sites de liaison de l’Ac-SDKP dans le myocarde, d’autres études seraient nécessaires pour caractériser entièrement et cloner les récepteurs de l’Ac-SDKP. Il demeure prometteur que l’Ac-SDKP ou ses analogues résistants à la dégradation puissent servir de nouveaux outils thérapeutiques contre les lésions et le dysfonctionnement du cœur, des vaisseaux et des reins utilisés seuls ou en association avec des agents pharmacothérapeutiques déjà établis contre les maladies cardiovasculaires. [Traduit par la Rédaction]
Keywords: Ac-SDKP; angiotensin-converting enzyme; cardiovasculaire; cardiovascular; enzyme de conversion de l’angiotensine; renal; rénal; thymosin beta 4; thymosine bêta 4.
Conflict of interest statement
Conflict of interest
The authors declare that there is no conflict of interest associated with this work.
Figures

References
-
- Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, et al. 1996. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J. Clin. Invest 97(3): 839–844. doi: 10.1172/JCI118484. PMID:8609242. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

