Human-Derived Organ-on-a-Chip for Personalized Drug Development
- PMID: 30854951
- PMCID: PMC6587585
- DOI: 10.2174/1381612825666190308150055
Human-Derived Organ-on-a-Chip for Personalized Drug Development
Abstract
To reduce the required capital and time investment in the development of new pharmaceutical agents, there is an urgent need for preclinical drug testing models that are predictive of drug response in human tissues or organs. Despite tremendous advancements and rigorous multistage screening of drug candidates involving computational models, traditional cell culture platforms, animal models and most recently humanized animals, there is still a large deficit in our ability to predict drug response in patient groups and overall attrition rates from phase 1 through phase 4 of clinical studies remain well above 90%. Organ-on-a-chip (OOC) platforms have proven potential in providing tremendous flexibility and robustness in drug screening and development by employing engineering techniques and materials. More importantly, in recent years, there is a clear upward trend in studies that utilize human-induced pluripotent stem cell (hiPSC) to develop personalized tissue or organ models. Additionally, integrated multiple organs on the single chip with increasingly more sophisticated representation of absorption, distribution, metabolism, excretion and toxicity (ADMET) process are being utilized to better understand drug interaction mechanisms in the human body and thus showing great potential to better predict drug efficacy and safety. In this review, we summarize these advances, highlighting studies that took the next step to clinical trials and research areas with the utmost potential and discuss the role of the OOCs in the overall drug discovery process at a preclinical and clinical stage, as well as outline remaining challenges.
Keywords: Organ-on-a-chip; drug development; human-derived induced pluripotent stem cells; microfluidic technology; personalized medicine; tissue engineering..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
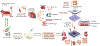



References
-
- DiMasi JA, Grabowski HG, Hansen RW JJohe. Innovation in the pharmaceutical industry: new estimates of R&D costs. 2016;47:20–33. - PubMed
-
- Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015., Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin Investig Drugs. 2017. 2017/06//;26, 26(6, 6):735,-9. - PMC - PubMed
-
- Begley CG, Ellis LM. Raise standards for preclinical cancer research. Nature. 2012. 2012/03/28/;483:531. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

