New insights into the links between hypoxia and iron homeostasis
- PMID: 30855332
- PMCID: PMC6467554
- DOI: 10.1097/MOH.0000000000000494
New insights into the links between hypoxia and iron homeostasis
Abstract
Purpose of review: This review outlines recent discoveries on the crosstalk between oxygen metabolism and iron homeostasis, focusing on the role of HIF-2 (hypoxia inducible factor-2) in the regulation of iron metabolism under physiopathological conditions.
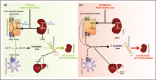
Recent findings: The importance of the hepcidin/ferroportin axis in the modulation of intestinal HIF-2 to regulate iron absorption has been recently highlighted. Latest advances also reveal a direct titration of the bone morphogenetic proteins by the erythroferrone contributing to liver hepcidin suppression to increase iron availability. Iron is recycled thanks to erythrophagocytosis of senescent erythrocytes by macrophages. Hemolysis is frequent in sickle cell anemia, leading to increased erythrophagocytosis responsible of the macrophage polarization shift. New findings assessed the effects of hemolysis on macrophage polarization in the tumor microenvironment.
Summary: Hypoxia signaling links erythropoiesis with iron homeostasis. The use of HIF stabilizing or inhibiting drugs are promising therapeutic approaches in iron-associated diseases.
Figures

References
-
- Beaumont C, Delaby C. Recycling iron in normal and pathological states. Semin Hematol 2009; 46:328–338. - PubMed
-
- Nairz M, Schroll A, Demetz E, et al. ’Ride on the ferrous wheel’ -- the cycle of iron in macrophages in health and disease. Immunobiology 2015; 220:280–294. - PubMed
-
- Armitage AE, Eddowes LA, Gileadi U, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood 2011; 118:4129–4139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

