Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review
- PMID: 30855527
- PMCID: PMC6727956
- DOI: 10.1097/CMR.0000000000000589
Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review
Abstract
Immune checkpoint inhibitors (ICIs), which target CTLA-4 or PD-(L)1 molecules, have shown impressive therapeutic results. Durable responses, however, are only observed in a segment of the patient population and must be offset against severe off-target immune toxicity and high costs. This calls for biomarkers that predict response during ICI treatment. Although many candidate biomarkers exist, as yet, there has been no systematic overview of biomarkers predictive during. Here, we provide a systematic review of the current literature of ICI treatment to establish an overview of candidate predictive biomarkers during ICI treatment in melanoma patients. We performed a systematic Medline search (2000-2018, 1 January) on biomarkers for survival or response to ICI treatment in melanoma patients. We retrieved 735 publications, of which 79 were finally included in this systematic review. Blood markers were largely studied for CTLA-4 ICI, whereas tumor tissue markers were analyzed for PD-(L)1 ICI. Blood cytology and soluble factors were more frequently correlated to overall survival (OS) than response, indicating their prognostic rather than predictive nature. An increase in tumor-infiltrating CD8 + T-cells and a decrease in regulatory T-cells were correlated to response, in addition to mutational load, neoantigen load, and immune-related gene expression. Immune-related adverse events were also associated frequently with a favorable response and OS. This review shows the great variety of potential biomarkers published to date, in an attempt to better understand response to ICI therapy; it also highlights the candidate markers for future research. The most promising biomarkers for response to ICI treatment are the occurrence of immune-related adverse events (especially vitiligo), lowering of lactate dehydrogenase, and increase in activated CD8 + and decrease in regulatory T-cells.
Figures
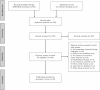
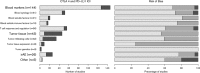


References
-
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement Ann Intern Med. 2009; 151:264–269 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

