Association of Piriform Cortex Resection With Surgical Outcomes in Patients With Temporal Lobe Epilepsy
- PMID: 30855662
- PMCID: PMC6490233
- DOI: 10.1001/jamaneurol.2019.0204
Association of Piriform Cortex Resection With Surgical Outcomes in Patients With Temporal Lobe Epilepsy
Abstract
Importance: A functional area associated with the piriform cortex, termed area tempestas, has been implicated in animal studies as having a crucial role in modulating seizures, but similar evidence is limited in humans.
Objective: To assess whether removal of the piriform cortex is associated with postoperative seizure freedom in patients with temporal lobe epilepsy (TLE) as a proof-of-concept for the relevance of this area in human TLE.
Design, setting, and participants: This cohort study used voxel-based morphometry and volumetry to assess differences in structural magnetic resonance imaging (MRI) scans in consecutive patients with TLE who underwent epilepsy surgery in a single center from January 1, 2005, through December 31, 2013. Participants underwent presurgical and postsurgical structural MRI and had at least 2 years of postoperative follow-up (median, 5 years; range, 2-11 years). Patients with MRI of insufficient quality were excluded. Findings were validated in 2 independent cohorts from tertiary epilepsy surgery centers. Study follow-up was completed on September 23, 2016, and data were analyzed from September 24, 2016, through April 24, 2018.
Exposures: Standard anterior temporal lobe resection.
Main outcomes and measures: Long-term postoperative seizure freedom.
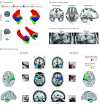
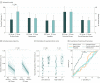
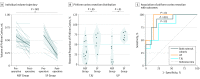
Results: In total, 107 patients with unilateral TLE (left-sided in 68; 63.6% women; median age, 37 years [interquartile range {IQR}, 30-45 years]) were included in the derivation cohort. Reduced postsurgical gray matter volumes were found in the ipsilateral piriform cortex in the postoperative seizure-free group (n = 46) compared with the non-seizure-free group (n = 61). A larger proportion of the piriform cortex was resected in the seizure-free compared with the non-seizure-free groups (median, 83% [IQR, 64%-91%] vs 52% [IQR, 32%-70%]; P < .001). The results were seen in left- and right-sided TLE and after adjusting for clinical variables, presurgical gray matter alterations, presurgical hippocampal volumes, and the proportion of white matter tract disconnection. Findings were externally validated in 2 independent cohorts (31 patients; left-sided TLE in 14; 54.8% women; median age, 41 years [IQR, 31-46 years]). The resected proportion of the piriform cortex was individually associated with seizure outcome after surgery (derivation cohort area under the curve, 0.80 [P < .001]; external validation cohorts area under the curve, 0.89 [P < .001]). Removal of at least half of the piriform cortex increased the odds of becoming seizure free by a factor of 16 (95% CI, 5-47; P < .001). Other mesiotemporal structures (ie, hippocampus, amygdala, and entorhinal cortex) and the overall resection volume were not associated with outcomes.
Conclusions and relevance: These results support the importance of resecting the piriform cortex in neurosurgical treatment of TLE and suggest that this area has a key role in seizure generation.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

