Cysteamine Protects Neurons from Mutant Huntingtin Toxicity
- PMID: 30856117
- PMCID: PMC7039181
- DOI: 10.3233/JHD-180312
Cysteamine Protects Neurons from Mutant Huntingtin Toxicity
Erratum in
-
Cysteamine Protects Neurons from Mutant Huntingtin Toxicity.J Huntingtons Dis. 2020;9(3):321. doi: 10.3233/JHD-200003. J Huntingtons Dis. 2020. PMID: 32623404 No abstract available.
Abstract
Background: The potential benefit of cysteamine for Huntington's disease has been demonstrated in HD animal models. Cysteamine and its derivate cystamine were shown to reduce neuropathology and prolong lifespan. Human studies have demonstrated safety, and suggestive results regarding efficacy. Despite all the studies available in vivo, there are only few in vitro studies, and the mechanism of action of cysteamine remains unclear.
Objective: The objective of this study is to assess the capacity of cysteamine for neuroprotection against mutant Huntingtin in vitro using cellular models of HD, and to provide initial data regarding mechanism of action.
Methods: We tested the neuroprotective properties of cysteamine in vitro in our primary neuron and iPSC models of HD.
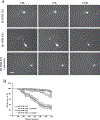
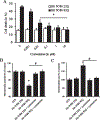
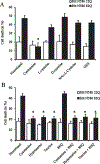
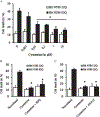
Results: Cysteamine showed a strong neuroprotective effect (EC50 = 7.1 nM) against mutant Htt-(aa-1-586 82Q) toxicity, in a nuclear condensation cell toxicity assay. Cysteamine also rescued mitochondrial changes induced by mutant Htt. Modulation of the levels of cysteine or glutathione failed to protect neurons, suggesting that cysteamine neuroprotection is not mediated through cysteine metabolism. Taurine and Hypotaurine, which are metabolites of cysteamine can protect neurons against Htt toxicity, but the inhibition of the enzyme converting cysteamine to hypotaurine does not block either protective activity, suggesting independent protective pathways. Cysteamine has been suggested to activate BDNF secretion; however, cysteamine protection was not blocked by BDNF pathway antagonists.
Conclusions: Cysteamine was strongly neuroprotective with relatively high potency. We demonstrated that the main neuroprotective pathways that have been proposed to be the mechanism of protection by cysteamine can all be blocked and still not prevent the neuroprotective effect. The results suggest the involvement of other yet-to-be-determined neuroprotective pathways.
Keywords: Huntington’s disease; cysteamine; huntingtin toxicity; neurodegeneration; neuroprotection; patient-derived induced pluripotent stem cells; primary neurons.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Figures



References
-
- McColgan P, Tabrizi SJ. Huntington’s disease: A clinical review. Eur J Neurol. 2018;25(1):24–34. - PubMed
-
- Besouw M, Masereeuw R, van den Heuvel L, Levtchenko E. Cysteamine: An old drug with new potential. Drug Discov Today. 2013;18(15–16):785–92. - PubMed
-
- Karpuj MV, Becher MW, Steinman L. Evidence for a role for transglutaminase in Huntington’s disease and the potential therapeutic implications. Neurochem Int. 2002;40(1):31–6. - PubMed
-
- Van Raamsdonk JM, Pearson J, Bailey CD, Rogers DA, Johnson GV, Hayden MR, et al. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95(1):210–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

