Modeling differentiation-state transitions linked to therapeutic escape in triple-negative breast cancer
- PMID: 30856168
- PMCID: PMC6428348
- DOI: 10.1371/journal.pcbi.1006840
Modeling differentiation-state transitions linked to therapeutic escape in triple-negative breast cancer
Erratum in
-
Correction: Modeling differentiation-state transitions linked to therapeutic escape in triple-negative breast cancer.PLoS Comput Biol. 2019 Oct 9;15(10):e1007441. doi: 10.1371/journal.pcbi.1007441. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31596847 Free PMC article.
Abstract
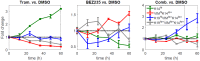
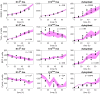
Drug resistance in breast cancer cell populations has been shown to arise through phenotypic transition of cancer cells to a drug-tolerant state, for example through epithelial-to-mesenchymal transition or transition to a cancer stem cell state. However, many breast tumors are a heterogeneous mixture of cell types with numerous epigenetic states in addition to stem-like and mesenchymal phenotypes, and the dynamic behavior of this heterogeneous mixture in response to drug treatment is not well-understood. Recently, we showed that plasticity between differentiation states, as identified with intracellular markers such as cytokeratins, is linked to resistance to specific targeted therapeutics. Understanding the dynamics of differentiation-state transitions in this context could facilitate the development of more effective treatments for cancers that exhibit phenotypic heterogeneity and plasticity. In this work, we develop computational models of a drug-treated, phenotypically heterogeneous triple-negative breast cancer (TNBC) cell line to elucidate the feasibility of differentiation-state transition as a mechanism for therapeutic escape in this tumor subtype. Specifically, we use modeling to predict the changes in differentiation-state transitions that underlie specific therapy-induced changes in differentiation-state marker expression that we recently observed in the HCC1143 cell line. We report several statistically significant therapy-induced changes in transition rates between basal, luminal, mesenchymal, and non-basal/non-luminal/non-mesenchymal differentiation states in HCC1143 cell populations. Moreover, we validate model predictions on cell division and cell death empirically, and we test our models on an independent data set. Overall, we demonstrate that changes in differentiation-state transition rates induced by targeted therapy can provoke distinct differentiation-state aggregations of drug-resistant cells, which may be fundamental to the design of improved therapeutic regimens for cancers with phenotypic heterogeneity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

