Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation
- PMID: 30856272
- PMCID: PMC6410951
- DOI: 10.1093/jnci/djy221
Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation
Abstract
Background: Barriers to cancer clinical trial participation have been the subject of frequent study, but the rate of trial participation has not changed substantially over time. Studies often emphasize patient-related barriers, but other types of barriers may have greater impact on trial participation. Our goal was to examine the magnitude of different domains of trial barriers by synthesizing prior research.
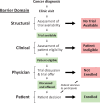
Methods: We conducted a systematic review and meta-analysis of studies that examined the trial decision-making pathway using a uniform framework to characterize and quantify structural (trial availability), clinical (eligibility), and patient/physician barrier domains. The systematic review utilized the PubMed, Google Scholar, Web of Science, and Ovid Medline search engines. We used random effects to estimate rates of different domains across studies, adjusting for academic vs community care settings.
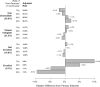
Results: We identified 13 studies (nine in academic and four in community settings) with 8883 patients. A trial was unavailable for patients at their institution 55.6% of the time (95% confidence interval [CI] = 43.7% to 67.3%). Further, 21.5% (95% CI = 10.9% to 34.6%) of patients were ineligible for an available trial, 14.8% (95% CI = 9.0% to 21.7%) did not enroll, and 8.1% (95% CI = 6.3% to 10.0%) enrolled. Rates of trial enrollment in academic (15.9% [95% CI = 13.8% to 18.2%]) vs community (7.0% [95% CI = 5.1% to 9.1%]) settings differed, but not rates of trial unavailability, ineligibility, or non-enrollment.
Conclusions: These findings emphasize the enormous need to address structural and clinical barriers to trial participation, which combined make trial participation unachievable for more than three of four cancer patients.
© The Author(s) 2019. Published by Oxford University Press.
Figures





References
-
- IOM (Institute of Medicine). Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington, DC: The National Academies Press; 2010. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. - PubMed
-
- Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117. - PubMed
-
- Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88(12):812–816. - PubMed
-
- Comis RL, Miller JD, Aldigé CR, Krebs L, Stoval E.. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21(5):830–835. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

