An Exfoliated Graphite-Based Electrochemical Immunosensor on a Dendrimer/Carbon Nanodot Platform for the Detection of Carcinoembryonic Antigen Cancer Biomarker
- PMID: 30857164
- PMCID: PMC6468524
- DOI: 10.3390/bios9010039
An Exfoliated Graphite-Based Electrochemical Immunosensor on a Dendrimer/Carbon Nanodot Platform for the Detection of Carcinoembryonic Antigen Cancer Biomarker
Abstract
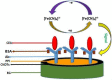
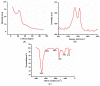
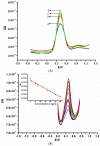
An electrochemical immunosensor for the quantification of carcinoembryonic antigen (CEA) using a nanocomposite of polypropylene imine dendrimer (PPI) and carbon nanodots (CNDTs) on an exfoliated graphite electrode (EG) is reported. The carbon nanodots were prepared by pyrolysis of oats. The nanocomposites (PPI and CNDTs) were characterized using X-ray powder diffraction (XRD), Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), high-resolution transmission electron microscopy (HRTEM) and scanning electron microscopy (SEM). The proposed immunosensor was prepared on an exfoliated graphite electrode sequentially by drop coating CNDTs, the electrodeposition of G2-PPI (generation 2 poly (propylene imine) dendrimer), the immobilization of anti-CEA on the modified electrode for 80 min at 35 °C, and dropping of bovine serum albumin (BSA) to minimize non-specific binding sites. Cyclic voltammetry was used to characterize each stage of the fabrication of the immunosensor. The proposed immunosensor detected CEA within a concentration range of 0.005 to 300 ng/mL with a detection limit of 0.00145 ng/mL by using differential pulse voltammetry (DPV). The immunosensor displayed good stability and was also selective in the presence of some interference species such as ascorbic acid, glucose, alpha-fetoprotein, prostate-specific antigen and human immunoglobulin. Furthermore, the fabricated immunosensor was applied in the quantification of CEA in a human serum sample, indicating its potential for real sample analysis.
Keywords: cancer; carcinoembryonic antigen; exfoliated graphite electrode; immunosensor; polypropylene imine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Huang J., Zhao L., Lei W., Wen W., Wang Y., Bao T., Xiong H. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018;99:28–33. doi: 10.1016/j.bios.2017.07.036. - DOI - PubMed
-
- Han J., Li Y., Feng J., Li M., Wang P., Chen Z., Dong Y. A novel sandwich-type immunosensor for detection of carcino-embryonic antigen using silver hybrid multiwalled carbon nanotubes/manganese dioxide. J. Electroanal. Chem. 2017;786:112–119. doi: 10.1016/j.jelechem.2017.01.021. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

