Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells
- PMID: 30857277
- PMCID: PMC6429456
- DOI: 10.3390/ijms20051198
Genotoxic Effects of Tributyltin and Triphenyltin Isothiocyanates, Cognate RXR Ligands: Comparison in Human Breast Carcinoma MCF 7 and MDA-MB-231 Cells
Abstract
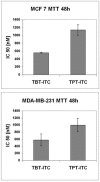
The cytotoxicity of two recently synthesized triorganotin isothiocyanate derivatives, nuclear retinoid X receptor ligands, was tested and compared in estrogen-receptor-positive MCF 7 and -negative MDA-MB-231 human breast carcinoma cell lines. A 48 h MTT assay indicated that tributyltin isothiocyanate (TBT-ITC) is more cytotoxic than triphenyltin isothiocyanate (TPT-ITC) in MCF 7 cells, and the same trend was observed in the MDA-MB-231 cell line. A comet assay revealed the presence of both crosslinks and increasing DNA damage levels after the 17 h treatment with both derivatives. Differences in cytotoxicity of TBT-ITC and TPT-ITC detected by FDA staining correspond to the MTT data, communicating more pronounced effects in MCF 7 than in the MDA-MB-231 cell line. Both derivatives were found to cause apoptosis, as shown by the mitochondrial membrane potential (MMP) depolarization and caspase-3/7 activation. The onset of caspase activation correlated with MMP dissipation and the total cytotoxicity more than with the amount of active caspases. In conclusion, our data suggest that the DNA damage induced by TBT-ITC and TPT-ITC treatment could underlie their cytotoxicity in the cell lines studied.
Keywords: DNA crosslinks; apoptosis; breast cancer; cytotoxicity; triorganotin isothiocyanates.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Nakanishi T., Nishikawa J., Hiromori Y., Yokoyama H., Koyanagi M., Takasuga S., Ishizaki J., Watanabe M., Isa S., Utoguchi N., et al. Trialkyltin compounds bind retinoid X receptor to alter human placental endocrine functions. Mol. Endocrinol. 2005;19:2502–2516. doi: 10.1210/me.2004-0397. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- APVV-15-0372/Agentúra na Podporu Výskumu a Vývoja
- 2/0084/16, 2/0092/16 and 1/0136/18/Scientific Grant Agency of the Ministry of Education of Slovak Republic and the Academy of Sciences
- 320/2018/FaF/IGA UVPS Brno
- TRANSMED, ITMS: 26240120008 and ITMS: 26240220071 and TRANSMED 2, ITMS: 26240120030/Research & Development Operational Programme funded by the ERDF
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

