Development of a web-based assessment tool that evaluates the meal situation when a child has a percutaneous endoscopic gastrostomy
- PMID: 30857527
- PMCID: PMC6410499
- DOI: 10.1186/s12887-019-1447-1
Development of a web-based assessment tool that evaluates the meal situation when a child has a percutaneous endoscopic gastrostomy
Abstract
Background: Children with cancer often suffer side effects from their treatment, for example nausea and vomiting, which can lead to malnutrition. If a child cannot eat orally, a percutaneous endoscopic gastrostomy (PEG) can improve his or her well-being, psychosocial development and growth by enabling the supply of nourishment and facilitating the administration of necessary medicines. Few data exist on children's comfort when using a PEG. The aim of this study was firstly to develop three versions of a web-based assessment tool in which children, families, and healthcare professionals would be able to register their observations and assessments for evaluating the meal situation when a child has a PEG and secondly to validate the content of the tool.
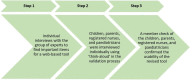
Methods: A qualitative design was chosen with purposive sampling of participants. Five children with cancer, five parents, five registered nurses and five paediatricians participated first in an interview and then in a member check of the web-based tool. The data were analysed with manifest qualitative content analysis.
Results: The results highlighted four categories of issues which needed to be revised in the web-based tool: words which were difficult for the participants to understand, items which contained several questions, items which needed to be split into more items to be answerable and the layout of the questionnaire. The web-based tool was revised according to the categories, and then a member check evaluated and finally confirmed the revisions.
Conclusions: A web-based tool may be able to evaluate the meal situation when a child with cancer has a PEG. The tool may be able to detect early failures of the PEG, facilitating early action from the healthcare professionals in supporting the child and his or her parents in their care of the PEG. In the long run, this web-based tool may also be able to increase the quality of care of children living with a PEG.
Keywords: Cancer; Child; Gastrostomy tube; Web tool.
Conflict of interest statement
Ethics approval and consent to participate
The Regional Ethical Review Board at the University of Gothenburg, Gothenburg, approved the study (Dnr: 312–14). Informed written and verbal consent was obtained from all the participants. Parental written and verbal consent was obtained for participants under 16 years of age. The study was conducted voluntarily, and all participants knew that they could withdraw their participation whenever they wanted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The protocol for a randomised-controlled trial of the evaluation of the tolerance and safety of early enteral nutrition in children after percutaneous endoscopic gastrostomy placement. (protocol version 09.01.2015).BMC Pediatr. 2016 Oct 7;16(1):163. doi: 10.1186/s12887-016-0705-8. BMC Pediatr. 2016. PMID: 27717336 Free PMC article. Clinical Trial.
-
Percutaneous endoscopic gastrostomy with jejunal extension plus percutaneous endoscopic gastrostomy (PEG-j plus PEG) in patients with gastric/duodenal cancer outlet obstruction.Arq Gastroenterol. 2015 Jan-Mar;52(1):72-5. doi: 10.1590/S0004-28032015000100015. Arq Gastroenterol. 2015. PMID: 26017087
-
Laparoscopic Gastrostomy Is Superior to Percutaneous Endoscopic Gastrostomy Tube Placement in Children Less Than 5 years of Age.J Laparoendosc Adv Surg Tech A. 2016 Jul;26(7):570-3. doi: 10.1089/lap.2016.0099. Epub 2016 Jun 6. J Laparoendosc Adv Surg Tech A. 2016. PMID: 27268954
-
Prophylactic percutaneous endoscopic gastrostomy tube placement in treatment of head and neck cancer: a comprehensive review and call for evidence-based medicine.JPEN J Parenter Enteral Nutr. 2011 May;35(3):365-74. doi: 10.1177/0148607110377097. JPEN J Parenter Enteral Nutr. 2011. PMID: 21527598 Review.
-
Enteral feeding and percutaneous endoscopic gastrostomy.Nurs Stand. 2004 Jan 28-Feb 3;18(20):41-3. doi: 10.7748/ns2004.01.18.20.41.c3536. Nurs Stand. 2004. PMID: 14976704 Review.
References
-
- Martinez-Costa C, Calderón C, Gómez-López L, Borraz S, Pedrón-Giner C. Satisfaction with gastrostomy feeding in caregivers of children with home enteral nutrition: application of the SAGA-8 questionnaire and analysis of involved factors. Nutritión Hospitalaria. 2013;28:1121–1128. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources