Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue
- PMID: 30857558
- PMCID: PMC6410510
- DOI: 10.1186/s40478-019-0686-6
Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue
Abstract
[F-18]-MK-6240, a novel tau positron emission tomography (PET) tracer recently discovered for the in vivo detection of neurofibrillary tangles, has the potential to improve diagnostic accuracy in the detection of Alzheimer disease. We have examined regional and substrate-specific binding patterns as well as possible off-target binding of this tracer on human brain tissue to advance towards its validation. We applied [F-18]-MK-6240 phosphor screen and high resolution autoradiography to postmortem samples from patients with a definite pathological diagnosis of Alzheimer disease, frontotemporal lobar degeneration-tau (Pick's disease, progressive supranuclear palsy and corticobasal degeneration), chronic traumatic encephalopathy, frontotemporal lobar degeneration-Tar DNA-binding protein 43 (TDP-43), dementia with Lewy bodies, cerebral amyloid angiopathy and elderly controls free of pathologic changes of neurodegenerative disease. We also directly compared the binding properties of [F-18]-MK-6240 and [F-18]-AV-1451 in human tissue, and examined potential nonspecific binding of both tau tracers to monoamine oxidases (MAO) by using autoradiography in the presence of selective monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B) inhibitors. Our data indicate that MK-6240 strongly binds to neurofibrillary tangles in Alzheimer disease but does not seem to bind to a significant extent to tau aggregates in non-Alzheimer tauopathies, suggesting that it may have a limited utility for the in vivo detection of these pathologies. There is no evidence of binding to lesions containing β-amyloid, α-synuclein or TDP-43. In addition, we identified MK-6240 strong off-target binding to neuromelanin and melanin-containing cells, and some weaker binding to areas of hemorrhage. These binding patterns are nearly identical to those previously reported by our group and others for [F-18]-AV-1451. Of note, [F-18]-MK-6240 and [F-18]-AV-1451 autoradiographic binding signals were only weakly displaced by competing concentrations of selective MAO-B inhibitor deprenyl but not by MAO-A inhibitor clorgyline, suggesting that MAO enzymes do not appear to be a significant binding target of any of these two tracers. Together these novel findings provide relevant insights for the correct interpretation of in vivo [F-18]-MK-6240 PET imaging.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Informed consent was obtained from all individual participants included in the study and according to institutional procedures for autopsy consents for post-mortem tissue.
Competing interests
Cinthya Agüero received research funding from the International Health Central America Institute, San Jose, Costa Rica.
Marc D. Normandin received research funding from NIH National Institute of Neurological Disorders and Stroke (U01NS086659) and NIH National Institute of Mental Health (R01MH100350).
Keith A. Johnson received research funding from NIH (grants R01 EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435 and R01 AG037497) and the Alzheimer’s Association (ZEN-10-174,210 K). Keith A. Johnson has served as paid consultant for Bayer, GE Healthcare, Janssen Alzheimer’s Immunotherapy, Siemens Medical Solutions, Genzyme, Novartis, Biogen, Roche, ISIS Pharma, AZTherapy, GEHC, Lundberg, and Abbvie. He is a site co-investigator for Lilly/Avid, Janssen Immunotherapy and Pfizer.
Matthew P. Frosch received research funding from the Massachusetts Alzheimer’s Disease Research Center (NIH AG005134).
Teresa Gómez-Isla received research funding from NIH National Institute on Aging (AG005134, AG036694 and AG061206). Teresa Gómez-Isla participated as speaker in an Eli Lilly and Company sponsored educational symposium and serves in an Eli Lilly Data Monitoring Committee. Eli Lilly and Company owns Avid, the company that created AV-1451, one of the PET compounds used in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






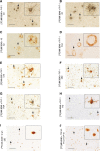
References
-
- Betthauser TJ, Cody KA, Zammit MD, Murali D, Converse AK, Barnhart TE, Stone CK, Rowley HA, Johnson SC, Christian BT (2018) In vivo characterization and quantification of neurofibrillary tau PET radioligand [(18)F]MK-6240 in humans from Alzheimer’s disease dementia to young controls. J Nucl Med. 10.2967/jnumed.118.209650. Epub ahead of print. PubMed PMID: 29777006. - PMC - PubMed
-
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM. Tau and ab imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:1–9. doi: 10.1126/scitranslmed.aaf2362. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01MH100350/NH/NIH HHS/United States
- R01 MH100350/MH/NIMH NIH HHS/United States
- R01 AG026484/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- AG061206/NH/NIH HHS/United States
- R21 AG038994/AG/NIA NIH HHS/United States
- R01 AG027435/AG/NIA NIH HHS/United States
- U01NS086659/NH/NIH HHS/United States
- R01 AG037497/AG/NIA NIH HHS/United States
- R01 EB014894/EB/NIBIB NIH HHS/United States
- P41 EB022544/EB/NIBIB NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- AG005134/NH/NIH HHS/United States
- U01 NS086659/NS/NINDS NIH HHS/United States
- RF1 AG061206/AG/NIA NIH HHS/United States
- R01 AG034556/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

