Reversal of a Treatment-Resistant, Depression-Related Brain State with the Kv7 Channel Opener Retigabine
- PMID: 30858110
- PMCID: PMC6511483
- DOI: 10.1016/j.neuroscience.2019.03.003
Reversal of a Treatment-Resistant, Depression-Related Brain State with the Kv7 Channel Opener Retigabine
Abstract
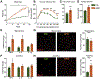
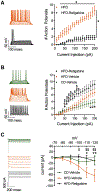
Neuroinflammation is associated with increased vulnerability to diverse psychiatric conditions, including treatment-resistant major depressive disorder (MDD). Here we assessed whether high fat diet (HFD) induced neuroinflammation may be suitable to model a treatment-resistant depressive-like brain state in mice. Male and female mice were fed a HFD for 18 weeks, followed by quantitation of glucose tolerance, inflammatory markers of brain tissue (TNFα, IL-6, IL-1β, Iba-1), neural excitability in the prelimbic cortex (PLC), as well as assessment of emotional reactivity and hedonic behavior in a battery of behavioral tests. In addition, we assessed the behavioral responsiveness of mice to fluoxetine, desipramine, ketamine, and the Kv7 channel opener and anticonvulsant retigabine. HFD exposure led to glucose intolerance and neuroinflammation in male mice, with similar but non-significant trends in females. Neuroinflammation of males was associated with anxious-depressive-like behavior and defects in working memory, along with neural hyperexcitability and increased Ih currents of pyramidal cells in the PLC. The behavioral changes were largely resistant to chronic treatment with fluoxetine and desipramine, as well as ketamine. By contrast, retigabine (also known as ezogabine) normalized neural excitability and Ih currents recorded from slices of HFD-treated animals and significantly ameliorated most of the behavioral impairments, without effects in control diet exposed animals. Thus, treatment resistant depressive-like brain states that are associated with chronic neuroinflammation may involve hyperexcitability of pyramidal neurons and may be effectively treated by retigabine.
Keywords: antidepressant; high fat diet; ketamine; neuroinflammation; neuronal hyperexcitability; obesity.
Copyright © 2019 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


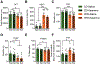
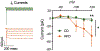

Similar articles
-
The novel dual-mechanism Kv7 potassium channel/TSPO receptor activator GRT-X is more effective than the Kv7 channel opener retigabine in the 6-Hz refractory seizure mouse model.Neuropharmacology. 2022 Feb 1;203:108884. doi: 10.1016/j.neuropharm.2021.108884. Epub 2021 Nov 14. Neuropharmacology. 2022. PMID: 34785163
-
The anticonvulsant retigabine suppresses neuronal KV2-mediated currents.Sci Rep. 2016 Oct 13;6:35080. doi: 10.1038/srep35080. Sci Rep. 2016. PMID: 27734968 Free PMC article.
-
Beyond Retigabine: Design, Synthesis, and Pharmacological Characterization of a Potent and Chemically Stable Neuronal Kv7 Channel Activator with Anticonvulsant Activity.J Med Chem. 2022 Aug 25;65(16):11340-11364. doi: 10.1021/acs.jmedchem.2c00911. Epub 2022 Aug 16. J Med Chem. 2022. PMID: 35972998 Free PMC article.
-
The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy.Epilepsia. 2012 Mar;53(3):412-24. doi: 10.1111/j.1528-1167.2011.03365.x. Epub 2012 Jan 5. Epilepsia. 2012. PMID: 22220513 Review.
-
Retigabine: chemical synthesis to clinical application.CNS Drug Rev. 2005 Spring;11(1):1-20. doi: 10.1111/j.1527-3458.2005.tb00033.x. CNS Drug Rev. 2005. PMID: 15867950 Free PMC article. Review.
Cited by
-
Impact of the KCNQ2/3 Channel Opener Ezogabine on Reward Circuit Activity and Clinical Symptoms in Depression: Results From a Randomized Controlled Trial.Am J Psychiatry. 2021 May 1;178(5):437-446. doi: 10.1176/appi.ajp.2020.20050653. Epub 2021 Mar 3. Am J Psychiatry. 2021. PMID: 33653118 Free PMC article. Clinical Trial.
-
Transcriptome signatures of the medial prefrontal cortex underlying GABAergic control of resilience to chronic stress exposure.Mol Psychiatry. 2025 May;30(5):2197-2209. doi: 10.1038/s41380-024-02832-x. Epub 2024 Nov 16. Mol Psychiatry. 2025. PMID: 39550415 Free PMC article.
-
Effects of the KCNQ (Kv7) Channel Opener Ezogabine on Resting-State Functional Connectivity of Striatal Brain Reward Regions, Depression, and Anhedonia in Major Depressive Disorder: Results From a Randomized Controlled Trial.Biol Psychiatry. 2025 Mar 4:S0006-3223(25)01011-X. doi: 10.1016/j.biopsych.2025.02.897. Online ahead of print. Biol Psychiatry. 2025. PMID: 40049579
-
Chemical modulation of Kv7 potassium channels.RSC Med Chem. 2021 Jan 14;12(4):483-537. doi: 10.1039/d0md00328j. eCollection 2021 Apr 28. RSC Med Chem. 2021. PMID: 34046626 Free PMC article. Review.
-
Kv7 Channels and Excitability Disorders.Handb Exp Pharmacol. 2021;267:185-230. doi: 10.1007/164_2021_457. Handb Exp Pharmacol. 2021. PMID: 33860384
References
-
- Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, Ryffel B, Apetoh L, Ghiringhelli F, Bullon P, Sanchez-Alcazar JA, Carrion AM, Cordero MD, 2016. Stress-Induced Depressive Behaviors Require a Functional NLRP3 Inflammasome. Mol Neurobiol 53, 4874–4882. doi: 10.1007/s12035-015-9408-7 - DOI - PubMed
-
- Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF, 2014. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67, 79–87. doi: 10.1016/j.nbd.2014.03.011 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

