Substitutions that lock and unlock the proton-coupled folate transporter (PCFT-SLC46A1) in an inward-open conformation
- PMID: 30858177
- PMCID: PMC6509503
- DOI: 10.1074/jbc.RA118.005533
Substitutions that lock and unlock the proton-coupled folate transporter (PCFT-SLC46A1) in an inward-open conformation
Abstract
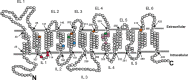
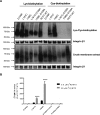
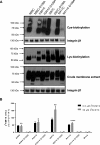
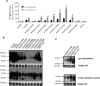
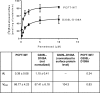
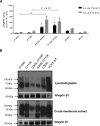
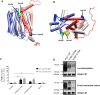
The proton-coupled folate transporter (PCFT) mediates intestinal absorption of folates and their transport from blood to cerebrospinal fluid across the choroid plexus. Substitutions at Asp-109 in the first intracellular loop between the first and second transmembrane domains (TMDs) abolish PCFT function, but protein expression and trafficking to the cell membrane are retained. Here, we used site-directed mutagenesis, the substituted-cysteine accessibility method, functional analyses, and homology modeling to determine whether the D109A substitution locks PCFT in one of its conformational states. Cys-substituted residues lining the PCFT aqueous translocation pathway and accessible in WT PCFT to the membrane-impermeable cysteine-biotinylation reagent, MTSEA-biotin, lost accessibility when introduced into the D109A scaffold. Substitutions at Gly-305 located exofacially within the eighth TMD, particularly with bulky residues, when introduced into the D109A scaffold largely restored function and MTSEA-biotin accessibility to Cys-substituted residues within the pathway. Likewise, Ser-196 substitution in the fifth TMD, predicted by homology modeling to be in proximity to Gly-305, also partially restored function found in solute transporters, is critical to oscillation of the carrier among its conformational states. Substitutions at Asp-109 and Gly-112 lock PCFT in an inward-open conformation, resulting in the loss of function. However, the integrity of the locked protein is preserved, indicated by the restoration of function after insertion of a second "unlocking" mutation. and accessibility. Similarly, the inactivating G112K substitution within the first intracellular loop was partially reactivated by introducing the G305L substitution. These data indicate that the first intracellular loop, with a sequence identical to "motif A" (GXXXDXXGR(R/K)).
Keywords: GXXXDXXGR(R/K); folate; folate malabsorption disease; membrane transport; protein motif; proton-coupled folate transporter (PCFT); solute carrier family 46 member 1 (SLC46A1); structural model; transporter.
© 2019 Aluri et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Qiu A., Jansen M., Sakaris A., Min S. H., Chattopadhyay S., Tsai E., Sandoval C., Zhao R., Akabas M. H., and Goldman I. D. (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127, 917–928 10.1016/j.cell.2006.09.041 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

