Cycloserine Population Pharmacokinetics and Pharmacodynamics in Patients with Tuberculosis
- PMID: 30858211
- PMCID: PMC6496076
- DOI: 10.1128/AAC.00055-19
Cycloserine Population Pharmacokinetics and Pharmacodynamics in Patients with Tuberculosis
Abstract
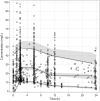
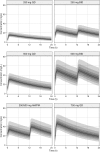
Limited pharmacokinetic/pharmacodynamic (PK/PD) data exist on cycloserine in tuberculosis (TB) patients. We pooled several studies into a large PK data set to estimate the population PK parameters for cycloserine in TB patients. We also performed simulations to provide insight into optimizing the dosing of cycloserine. TB patients were included from Georgia, Bangladesh, and four U.S. sites. Monolix and mlxR package were used for population PK modeling and simulation. We used PK/PD targets for time above MIC of ≥30% and ≥64%, representing bactericidal activity and 80% of the maximum kill, to calculate the probability of target attainment (PTA). Optimal PK/PD breakpoints were defined as the highest MIC to achieve ≥90% of PTA. Data from 247 subjects, including 205 patients with drug-resistant TB, were included. The data were best described by a one-compartment model. In most cases, the PK/PD breakpoints for the simulated regimens were similar for both PK/PD targets. Higher PTA were achieved as the total daily dose was increased. The highest PK/PD breakpoint that resulted from the use of 250 mg dosages was 16 mg/liter. For MICs of >16 mg/liter, doses of at least 500 mg three times daily or 750 mg twice daily were needed. In conclusion, the current dosing for cycloserine, 250 to 500 mg once or twice daily, is not sufficient for MICs of >16mg/liter. Further studies are needed regarding the efficacy and tolerability of daily doses of >1,000 mg. Dividing the dose minimally affected the PK/PD breakpoints while optimizing exposure, which can potentially reduce adverse drug effects.
Keywords: cycloserine; drug-resistant tuberculosis; pharmacodynamics; pharmacokinetics; target attainment.
Copyright © 2019 Alghamdi et al.
Figures

References
-
- World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2018. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

