LRG1 Promotes Diabetic Kidney Disease Progression by Enhancing TGF- β-Induced Angiogenesis
- PMID: 30858225
- PMCID: PMC6442349
- DOI: 10.1681/ASN.2018060599
LRG1 Promotes Diabetic Kidney Disease Progression by Enhancing TGF- β-Induced Angiogenesis
Abstract
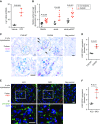
Background: Glomerular endothelial dysfunction and neoangiogenesis have long been implicated in the pathogenesis of diabetic kidney disease (DKD). However, the specific molecular pathways contributing to these processes in the early stages of DKD are not well understood. Our recent transcriptomic profiling of glomerular endothelial cells identified a number of proangiogenic genes that were upregulated in diabetic mice, including leucine-rich α-2-glycoprotein 1 (LRG1). LRG1 was previously shown to promote neovascularization in mouse models of ocular disease by potentiating endothelial TGF-β/activin receptor-like kinase 1 (ALK1) signaling. However, LRG1's role in the kidney, particularly in the setting of DKD, has been unclear.
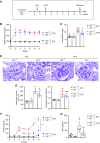
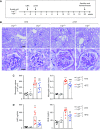
Methods: We analyzed expression of LRG1 mRNA in glomeruli of diabetic kidneys and assessed its localization by RNA in situ hybridization. We examined the effects of genetic ablation of Lrg1 on DKD progression in unilaterally nephrectomized, streptozotocin-induced diabetic mice at 12 and 20 weeks after diabetes induction. We also assessed whether plasma LRG1 was associated with renal outcome in patients with type 2 diabetes.
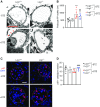
Results: LRG1 localized predominantly to glomerular endothelial cells, and its expression was elevated in the diabetic kidneys. LRG1 ablation markedly attenuated diabetes-induced glomerular angiogenesis, podocyte loss, and the development of diabetic glomerulopathy. These improvements were associated with reduced ALK1-Smad1/5/8 activation in glomeruli of diabetic mice. Moreover, increased plasma LRG1 was associated with worse renal outcome in patients with type 2 diabetes.
Conclusions: These findings identify LRG1 as a potential novel pathogenic mediator of diabetic glomerular neoangiogenesis and a risk factor in DKD progression.
Keywords: TGF-beta; diabetic nephropathy; glomerular endothelial cells; proteinuria.
Copyright © 2019 by the American Society of Nephrology.
Figures




References
-
- US Renal Data System : Annual Data Report: Atlas of End-Stage-Renal-Disease in the United States, 2011. Available at : https://www.usrds.org/atlas11.aspx. Accessed February 24, 2019
-
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. .: Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. .: RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 - PubMed
-
- de Zeeuw D, Heerspink HJL: Unmet need in diabetic nephropathy: Failed drugs or trials? Lancet Diabetes Endocrinol 4: 638–640, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL085757/HL/NHLBI NIH HHS/United States
- I01 BX000345/BX/BLRD VA/United States
- U01 DK106962/DK/NIDDK NIH HHS/United States
- R01 DK096549/DK/NIDDK NIH HHS/United States
- P01 DK056492/DK/NIDDK NIH HHS/United States
- U01 DK082185/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- P30 DK081943/DK/NIDDK NIH HHS/United States
- K23 DK107908/DK/NIDDK NIH HHS/United States
- R01 DK117913/DK/NIDDK NIH HHS/United States
- R01 DK078897/DK/NIDDK NIH HHS/United States
- R01 DK109683/DK/NIDDK NIH HHS/United States
- R01 DK088541/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

