Hydroxyurea reduces cerebral metabolic stress in patients with sickle cell anemia
- PMID: 30858231
- PMCID: PMC6543515
- DOI: 10.1182/blood-2018-09-876318
Hydroxyurea reduces cerebral metabolic stress in patients with sickle cell anemia
Abstract
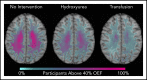
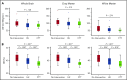
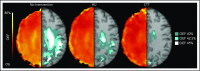
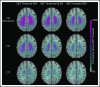
Chronic transfusion therapy (CTT) prevents stroke in selected patients with sickle cell anemia (SCA). We have shown that CTT mitigates signatures of cerebral metabolic stress, reflected by elevated oxygen extraction fraction (OEF), which likely drives stroke risk reduction. The region of highest OEF falls within the border zone, where cerebral blood flow (CBF) nadirs; OEF in this region was reduced after CTT. The neuroprotective efficacy of hydroxyurea (HU) remains unclear. To test our hypothesis that patients receiving HU therapy have lower cerebral metabolic stress compared with patients not receiving disease-modifying therapy, we prospectively obtained brain magnetic resonance imaging scans with voxel-wise measurements of CBF and OEF in 84 participants with SCA who were grouped by therapy: no disease-modifying therapy, HU, or CTT. There was no difference in whole-brain CBF among the 3 cohorts (P = .148). However, whole-brain OEF was significantly different (P < .001): participants without disease-modifying therapy had the highest OEF (median 42.9% [interquartile range (IQR) 39.1%-49.1%]), followed by HU treatment (median 40.7% [IQR 34.9%-43.6%]), whereas CTT treatment had the lowest values (median 35.3% [IQR 32.2%-38.9%]). Moreover, the percentage of white matter at highest risk for ischemia, defined by OEF greater than 40% and 42.5%, was lower in the HU cohort compared with the untreated cohort (P = .025 and P = .034 respectively), but higher compared with the CTT cohort (P = .018 and P = .029 respectively). We conclude that HU may offer neuroprotection by mitigating cerebral metabolic stress in patients with SCA, but not to the same degree as CTT.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.E.F. declares equity ownership in Proclara Biosciences. M.L.H. declares research funding from Global Blood Therapeutics; spouse employment at Pfizer, Inc.; and scientific advisory board membership in the Sickle Cell Transplant Alliance for Research. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Brain O2 reserve in sickle cell disease.Blood. 2019 May 30;133(22):2356-2358. doi: 10.1182/blood-2019-04-901124. Blood. 2019. PMID: 31147375 Free PMC article.
References
-
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. . Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288-294. - PubMed
-
- Adams R, McKie V, Nichols F, et al. . The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326(9):605-610. - PubMed
-
- Adams RJ, McKie VC, Carl EM, et al. . Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42(5):699-704. - PubMed
-
- Adams RJ, McKie VC, Hsu L, et al. . Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5-11. - PubMed
-
- Wang WC, Gallagher DM, Pegelow CH, et al. . Multicenter comparison of magnetic resonance imaging and transcranial Doppler ultrasonography in the evaluation of the central nervous system in children with sickle cell disease. J Pediatr Hematol Oncol. 2000;22(4):335-339. - PubMed

