Characterization of the Vibrio cholerae Phage Shock Protein Response
- PMID: 30858296
- PMCID: PMC6597379
- DOI: 10.1128/JB.00761-18
Characterization of the Vibrio cholerae Phage Shock Protein Response
Abstract
The phage shock protein (Psp) system is a stress response pathway that senses and responds to inner membrane damage. The genetic components of the Psp system are present in several clinically relevant Gram-negative bacteria, including Vibrio cholerae However, most of the current knowledge about the Psp response stems from in vitro studies in Escherichia coli and Yersinia enterocolitica In fact, the Psp response in V. cholerae has remained completely uncharacterized. In this study, we demonstrate that V. cholerae does have a functional Psp response system. We found that overexpression of GspD (EpsD), the type II secretion system secretin, induces the Psp response, whereas other V. cholerae secretins do not. In addition, we have identified several environmental conditions that induce this stress response. Our studies on the genetic regulation and induction of the Psp system in V. cholerae suggest that the key regulatory elements are conserved with those of other Gram-negative bacteria. While a psp null strain is fully capable of colonizing the infant mouse intestine, it exhibits a colonization defect in a zebrafish model, indicating that this response may be important for disease transmission in the environment. Overall, these studies provide an initial understanding of a stress response pathway that has not been previously investigated in V. choleraeIMPORTANCEVibrio cholerae leads a dual life cycle, as it can exist in the aquatic environment and colonize the human small intestine. In both life cycles, V. cholerae encounters a variety of stressful conditions, including fluctuating pH and temperature and exposure to other agents that may negatively affect cell envelope homeostasis. The phage shock protein (Psp) response is required to sense and respond to such insults in other bacteria but has remained unstudied in V. cholerae Interestingly, the Psp system has protein homologs, principally, PspA, in a number of bacterial clades as well as in archaea and plants. Therefore, our findings not only fill a gap in knowledge about an unstudied extracytoplasmic stress response in V. cholerae, but also may have far-reaching implications.
Keywords: Psp; Vibrio cholerae; cholera; stress response.
Copyright © 2019 American Society for Microbiology.
Figures



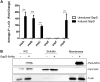



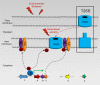
References
-
- Seo J, Savitzky DC, Ford E, Darwin AJ. 2007. Global analysis of tolerance to secretin-induced stress in Yersinia enterocolitica suggests that the phage-shock-protein system may be a remarkably self-contained stress response. Mol Microbiol 65:714–727. doi: 10.1111/j.1365-2958.2007.05821.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

