σB Inhibits Poly- N-Acetylglucosamine Exopolysaccharide Synthesis and Biofilm Formation in Staphylococcus aureus
- PMID: 30858304
- PMCID: PMC6509662
- DOI: 10.1128/JB.00098-19
σB Inhibits Poly- N-Acetylglucosamine Exopolysaccharide Synthesis and Biofilm Formation in Staphylococcus aureus
Abstract
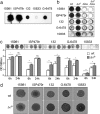
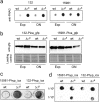
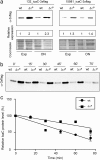
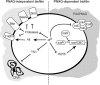
Staphylococcus aureus clinical strains are able to produce at least two distinct types of biofilm matrixes: biofilm matrixes made of the polysaccharide intercellular adhesin (PIA) or poly-N-acetylglucosamine (PNAG), whose synthesis is mediated by the icaADBC locus, and biofilm matrixes built of proteins (polysaccharide independent). σB is a conserved alternative sigma factor that regulates the expression of more than 100 genes in response to changes in environmental conditions. While numerous studies agree that σB is required for polysaccharide-independent biofilms, controversy persists over the role of σB in the regulation of PIA/PNAG-dependent biofilm development. Here, we show that genetically unrelated S. aureus σB-deficient strains produced stronger biofilms under both static and flow conditions and accumulated higher levels of PIA/PNAG exopolysaccharide than their corresponding wild-type strains. The increased accumulation of PIA/PNAG in the σB mutants correlated with a greater accumulation of the IcaC protein showed that it was not due to adjustments in icaADBC operon transcription and/or icaADBC mRNA stability. Overall, our results reveal that in the presence of active σB, the turnover of Ica proteins is accelerated, reducing the synthesis of PIA/PNAG exopolysaccharide and consequently the PIA/PNAG-dependent biofilm formation capacity.IMPORTANCE Due to its multifaceted lifestyle, Staphylococcus aureus needs a complex regulatory network to connect environmental signals with cellular physiology. One particular transcription factor, named σB (SigB), is involved in the general stress response and the expression of virulence factors. For many years, great confusion has existed about the role of σB in the regulation of the biofilm lifestyle in S. aureus Our study demonstrated that σB is not necessary for exopolysaccharide-dependent biofilms and, even more, that S. aureus produces stronger biofilms in the absence of σB The increased accumulation of exopolysaccharide correlates with higher stability of the proteins responsible for its synthesis. The present findings reveal an additional regulatory layer to control biofilm exopolysaccharide synthesis under stress conditions.
Keywords: Biofilm; PNAG; SigB; Staphylococcus aureus; ica operon.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Elucidating the crucial role of poly N-acetylglucosamine from Staphylococcus aureus in cellular adhesion and pathogenesis.PLoS One. 2015 Apr 15;10(4):e0124216. doi: 10.1371/journal.pone.0124216. eCollection 2015. PLoS One. 2015. PMID: 25876106 Free PMC article.
-
Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects.Front Cell Infect Microbiol. 2015 Feb 10;5:7. doi: 10.3389/fcimb.2015.00007. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25713785 Free PMC article. Review.
-
Phase variation of poly-N-acetylglucosamine expression in Staphylococcus aureus.PLoS Pathog. 2014 Jul 31;10(7):e1004292. doi: 10.1371/journal.ppat.1004292. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25077798 Free PMC article.
-
SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus.Mol Microbiol. 2003 May;48(4):1075-87. doi: 10.1046/j.1365-2958.2003.03493.x. Mol Microbiol. 2003. PMID: 12753197
-
ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus.FEMS Microbiol Lett. 2007 May;270(2):179-88. doi: 10.1111/j.1574-6968.2007.00688.x. Epub 2007 Apr 10. FEMS Microbiol Lett. 2007. PMID: 17419768 Review.
Cited by
-
Targeting Candida albicans in dual-species biofilms with antifungal treatment reduces Staphylococcus aureus and MRSA in vitro.PLoS One. 2021 Apr 8;16(4):e0249547. doi: 10.1371/journal.pone.0249547. eCollection 2021. PLoS One. 2021. PMID: 33831044 Free PMC article.
-
Treatment of periprosthetic joint infections guided by minimum biofilm eradication concentration (MBEC) in addition to minimum inhibitory concentration (MIC): protocol for a prospective randomised clinical trial.BMJ Open. 2022 Sep 15;12(9):e058168. doi: 10.1136/bmjopen-2021-058168. BMJ Open. 2022. PMID: 36109038 Free PMC article.
-
Lipophilic Extracts of Portulaca oleracea L.: Analysis of Bioactive Fatty Acids Targeting Microbial and Cancer Pathways.Pharmaceuticals (Basel). 2025 Apr 17;18(4):587. doi: 10.3390/ph18040587. Pharmaceuticals (Basel). 2025. PMID: 40284022 Free PMC article.
-
Within-host evolution of bovine Staphylococcus aureus selects for a SigB-deficient pathotype characterized by reduced virulence but enhanced proteolytic activity and biofilm formation.Sci Rep. 2019 Sep 17;9(1):13479. doi: 10.1038/s41598-019-49981-6. Sci Rep. 2019. PMID: 31530887 Free PMC article.
-
A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism.Antibiotics (Basel). 2022 Dec 22;12(1):12. doi: 10.3390/antibiotics12010012. Antibiotics (Basel). 2022. PMID: 36671212 Free PMC article. Review.
References
-
- Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184:5457–5467. doi:10.1128/JB.184.19.5457-5467.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

