Cardiac phenotype in mouse models of systemic autoimmunity
- PMID: 30858306
- PMCID: PMC6451423
- DOI: 10.1242/dmm.036947
Cardiac phenotype in mouse models of systemic autoimmunity
Abstract
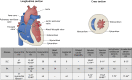
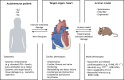
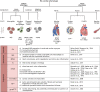
Patients suffering from systemic autoimmune diseases are at significant risk of cardiovascular complications. This can be due to systemically increased levels of inflammation leading to accelerated atherosclerosis, or due to direct damage to the tissues and cells of the heart. Cardiac complications include an increased risk of myocardial infarction, myocarditis and dilated cardiomyopathy, valve disease, endothelial dysfunction, excessive fibrosis, and bona fide autoimmune-mediated tissue damage by autoantibodies or auto-reactive cells. There is, however, still a considerable need to better understand how to diagnose and treat cardiac complications in autoimmune patients. A range of inducible and spontaneous mouse models of systemic autoimmune diseases is available for mechanistic and therapeutic studies. For this Review, we systematically collated information on the cardiac phenotype in the most common inducible, spontaneous and engineered mouse models of systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis. We also highlight selected lesser-known models of interest to provide researchers with a decision framework to choose the most suitable model for their study of heart involvement in systemic autoimmunity.
Keywords: Heart disease; Heart failure; Mouse model; Myocarditis; SLE; Systemic autoimmunity.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Systemic autoimmunity induced by the TLR7/8 agonist Resiquimod causes myocarditis and dilated cardiomyopathy in a new mouse model of autoimmune heart disease.Dis Model Mech. 2017 Mar 1;10(3):259-270. doi: 10.1242/dmm.027409. Dis Model Mech. 2017. PMID: 28250051 Free PMC article.
-
Cardiac Autoimmunity: Myocarditis.Adv Exp Med Biol. 2017;1003:187-221. doi: 10.1007/978-3-319-57613-8_10. Adv Exp Med Biol. 2017. PMID: 28667560 Free PMC article. Review.
-
Myosin-primed tolerogenic dendritic cells ameliorate experimental autoimmune myocarditis.Cardiovasc Res. 2014 Feb 1;101(2):203-10. doi: 10.1093/cvr/cvt246. Epub 2013 Nov 4. Cardiovasc Res. 2014. PMID: 24189626
-
Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy.Circ Res. 2009 Oct 23;105(9):912-20. doi: 10.1161/CIRCRESAHA.109.199802. Epub 2009 Sep 17. Circ Res. 2009. PMID: 19762681
-
Inflammatory dilated cardiomyopathy (DCMI).Herz. 2005 Sep;30(6):535-44. doi: 10.1007/s00059-005-2730-5. Herz. 2005. PMID: 16170686 Review.
Cited by
-
Toward the Effective Bioengineering of a Pathological Tissue for Cardiovascular Disease Modeling: Old Strategies and New Frontiers for Prevention, Diagnosis, and Therapy.Front Cardiovasc Med. 2021 Mar 4;7:591583. doi: 10.3389/fcvm.2020.591583. eCollection 2020. Front Cardiovasc Med. 2021. PMID: 33748193 Free PMC article. Review.
-
Inflammation-induced left ventricular fibrosis is partially mediated by tumor necrosis factor-α.Physiol Rep. 2021 Nov;9(21):e15062. doi: 10.14814/phy2.15062. Physiol Rep. 2021. PMID: 34713972 Free PMC article.
-
Quantitative assessment of left ventricular systolic function in patients with systemic lupus erythematosus: a non-invasive pressure-strain loop technique.Quant Imaging Med Surg. 2022 Jun;12(6):3170-3183. doi: 10.21037/qims-21-951. Quant Imaging Med Surg. 2022. PMID: 35655829 Free PMC article.
-
Chronic inflammatory diseases, myocardial function and cardioprotection.Br J Pharmacol. 2020 Dec;177(23):5357-5374. doi: 10.1111/bph.14975. Epub 2020 Feb 8. Br J Pharmacol. 2020. PMID: 31943142 Free PMC article. Review.
-
Characterization of acute TLR-7 agonist-induced hemorrhagic myocarditis in mice by multiparametric quantitative cardiac magnetic resonance imaging.Dis Model Mech. 2019 Aug 16;12(8):dmm040725. doi: 10.1242/dmm.040725. Dis Model Mech. 2019. PMID: 31324689 Free PMC article.

