Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array
- PMID: 30858401
- PMCID: PMC6411890
- DOI: 10.1038/s41598-019-40128-1
Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array
Abstract
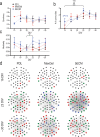
The brain's extracellular matrix (ECM) is a macromolecular network composed of glycosaminoglycans, proteoglycans, glycoproteins, and fibrous proteins. In vitro studies often use purified ECM proteins for cell culture coatings, however these may not represent the molecular complexity and heterogeneity of the brain's ECM. To address this, we compared neural network activity (over 30 days in vitro) from primary neurons co-cultured with glia grown on ECM coatings from decellularized brain tissue (bECM) or MaxGel, a non-tissue-specific ECM. Cells were grown on a multi-electrode array (MEA) to enable noninvasive long-term interrogation of neuronal networks. In general, the presence of ECM accelerated the formation of networks without affecting the inherent network properties. However, specific features of network activity were dependent on the type of ECM: bECM enhanced network activity over a greater region of the MEA whereas MaxGel increased network burst rate associated with robust synaptophysin expression. These differences in network activity were not attributable to cellular composition, glial proliferation, or astrocyte phenotypes, which remained constant across experimental conditions. Collectively, the addition of ECM to neuronal cultures represents a reliable method to accelerate the development of mature neuronal networks, providing a means to enhance throughput for routine evaluation of neurotoxins and novel therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. - PubMed
-
- Blau A. Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Current Opinion in Colloid & Interface Science. 2013;18:481–492. doi: 10.1016/j.cocis.2013.07.005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

