Physical plasma-triggered ROS induces tumor cell death upon cleavage of HSP90 chaperone
- PMID: 30858416
- PMCID: PMC6412052
- DOI: 10.1038/s41598-019-38580-0
Physical plasma-triggered ROS induces tumor cell death upon cleavage of HSP90 chaperone
Abstract
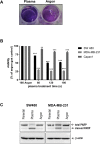
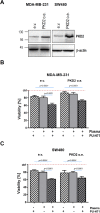
HSP90 is a ubiquitously expressed molecular chaperone implicated in the correct folding and maturation of a plethora of proteins including protein kinases and transcription factors. While disruption of chaperone activity was associated with augmented cancer cell death and decreased tumor growth both in vitro and in vivo, the regulation of HSP90 is not clearly understood. Here we report that treatment of cancer cells with cold physical plasma, an emerging and less aggressive tumor therapy, resulted in ROS generation which subsequently triggered the cleavage of HSP90. Notably, cleavage of HSP90 was followed by the degradation of PKD2, a crucial regulator of tumor growth and angiogenesis. Pre-sensitization of cancer cells with subliminal doses of PU-H71, an HSP90 inhibitor currently under clinical evaluation, followed by treatment with cold-plasma, synergistically and negatively impacted on the viability of cancer cells. Taken together, cold-plasma can be used in conjunction with pharmacologic treatment in order to target the expression and activity of HSP90 and the downstream client proteins implicated in various cancer cell capabilities.
Conflict of interest statement
Memorial Sloan-Kettering Cancer Center holds the intellectual rights to PU-H71. Samus Therapeutics, of which G. Chiosis has partial ownership, has licensed PU-H71. The corresponding authors are responsible for submitting on behalf of all authors of the paper.
Figures




Similar articles
-
Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2.Carcinogenesis. 2020 Oct 15;41(10):1421-1431. doi: 10.1093/carcin/bgaa001. Carcinogenesis. 2020. PMID: 31917403 Free PMC article.
-
Proteasome inhibitor-induced cleavage of HSP90 is mediated by ROS generation and caspase 10-activation in human leukemic cells.Redox Biol. 2017 Oct;13:470-476. doi: 10.1016/j.redox.2017.07.010. Epub 2017 Jul 12. Redox Biol. 2017. PMID: 28715732 Free PMC article.
-
Vorinostat enhances gefitinib‑induced cell death through reactive oxygen species‑dependent cleavage of HSP90 and its clients in non‑small cell lung cancer with the EGFR mutation.Oncol Rep. 2019 Jan;41(1):525-533. doi: 10.3892/or.2018.6814. Epub 2018 Oct 22. Oncol Rep. 2019. PMID: 30365122
-
[Molecular chaperone HSP90 as a novel target for cancer chemotherapy].Nihon Yakurigaku Zasshi. 2003 Jan;121(1):33-42. doi: 10.1254/fpj.121.33. Nihon Yakurigaku Zasshi. 2003. PMID: 12617036 Review. Japanese.
-
Cdc37 goes beyond Hsp90 and kinases.Cell Stress Chaperones. 2003 Summer;8(2):114-9. doi: 10.1379/1466-1268(2003)008<0114:cgbhak>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627196 Free PMC article. Review.
Cited by
-
Genome-Wide Comparison of the Target Genes of the Reactive Oxygen Species and Non-Reactive Oxygen Species Constituents of Cold Atmospheric Plasma in Cancer Cells.Cancers (Basel). 2020 Sep 16;12(9):2640. doi: 10.3390/cancers12092640. Cancers (Basel). 2020. PMID: 32947888 Free PMC article.
-
Differential Sensitivity of Melanoma Cells and Their Non-Cancerous Counterpart to Cold Atmospheric Plasma-Induced Reactive Oxygen and Nitrogen Species.Int J Mol Sci. 2022 Nov 15;23(22):14092. doi: 10.3390/ijms232214092. Int J Mol Sci. 2022. PMID: 36430569 Free PMC article.
-
Optimization of the Treatment of Squamous Cell Carcinoma Cells by Combining Photodynamic Therapy with Cold Atmospheric Plasma.Int J Mol Sci. 2024 Oct 8;25(19):10808. doi: 10.3390/ijms251910808. Int J Mol Sci. 2024. PMID: 39409136 Free PMC article.
-
Anticancer Effects of Cold Atmospheric Plasma in Canine Osteosarcoma Cells.Int J Mol Sci. 2020 Jun 26;21(12):4556. doi: 10.3390/ijms21124556. Int J Mol Sci. 2020. PMID: 32604902 Free PMC article.
-
Non-thermal plasma modulates cellular markers associated with immunogenicity in a model of latent HIV-1 infection.PLoS One. 2021 Mar 1;16(3):e0247125. doi: 10.1371/journal.pone.0247125. eCollection 2021. PLoS One. 2021. PMID: 33647028 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

