TouchScreen-based phenotyping: altered stimulus/reward association and lower perseveration to gain a reward in mu opioid receptor knockout mice
- PMID: 30858487
- PMCID: PMC6411729
- DOI: 10.1038/s41598-019-40622-6
TouchScreen-based phenotyping: altered stimulus/reward association and lower perseveration to gain a reward in mu opioid receptor knockout mice
Abstract
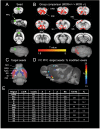
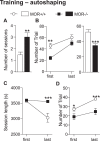
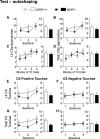
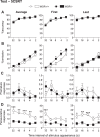
While the contribution of Mu Opioid Receptors (MORs) to hedonic aspects of reward processing is well-established, the notion that these receptors may also regulate motivation to gain a reward, and possibly other related cognitive dimensions, has been less investigated. The prefrontal cortex (PFC) is a critical site for these processes. Our previous functional magnetic resonance imaging study found alterations of functional connectivity (FC) in reward/aversion networks in MOR knockout mice. Here we pursued voxelwise seed-based FC analyses using the same dataset with a focus on the PFC. We observed significant reduction of PFC FC in mutant mice, predominantly with the nucleus accumbens, supporting the notion of altered reward-driven top-down controls. We tested motivation for palatable food in a classical operant self-administration paradigm, and found delayed performance for mutant mice. We then evaluated motivational and cognitive abilities of MOR knockout mice in TouchScreen-based behavioral tests. Learning was delayed and stimulus/reward association was impaired, suggesting lower hedonic reward value and reduced motivation. Perseverative responses were decreased, while discriminatory behavior and attention were unchanged, indicative of increased inhibitory controls with otherwise intact cognitive performance. Together, our data suggest that MORs contribute to enhance reward-seeking and facilitate perseverative behaviors. The possibility that MOR blockade could reduce maladaptive compulsivity deserves further investigation in addiction and self-control disorder research.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

