Glycosylation in health and disease
- PMID: 30858582
- PMCID: PMC6590709
- DOI: 10.1038/s41581-019-0129-4
Glycosylation in health and disease
Erratum in
-
Publisher Correction: Glycosylation in health and disease.Nat Rev Nephrol. 2025 Mar;21(3):216. doi: 10.1038/s41581-024-00923-0. Nat Rev Nephrol. 2025. PMID: 39668240 No abstract available.
Abstract
The glycome describes the complete repertoire of glycoconjugates composed of carbohydrate chains, or glycans, that are covalently linked to lipid or protein molecules. Glycoconjugates are formed through a process called glycosylation and can differ in their glycan sequences, the connections between them and their length. Glycoconjugate synthesis is a dynamic process that depends on the local milieu of enzymes, sugar precursors and organelle structures as well as the cell types involved and cellular signals. Studies of rare genetic disorders that affect glycosylation first highlighted the biological importance of the glycome, and technological advances have improved our understanding of its heterogeneity and complexity. Researchers can now routinely assess how the secreted and cell-surface glycomes reflect overall cellular status in health and disease. In fact, changes in glycosylation can modulate inflammatory responses, enable viral immune escape, promote cancer cell metastasis or regulate apoptosis; the composition of the glycome also affects kidney function in health and disease. New insights into the structure and function of the glycome can now be applied to therapy development and could improve our ability to fine-tune immunological responses and inflammation, optimize the performance of therapeutic antibodies and boost immune responses to cancer. These examples illustrate the potential of the emerging field of 'glycomedicine'.
Conflict of interest statement
Competing interests
J.N. and M.B.R. are co-founders of Reliant Glycosciences, LLC. The other authors declare no competing interests.
Figures
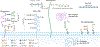


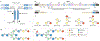
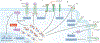


References
-
- Laine RA A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 10(12) structures for a reducing hexasaccharide: the Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4, 759–767 (1994). - PubMed
-
- Spiro RG Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12, 43R–56R (2002). - PubMed
-
- Gagneux P & Varki A Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9, 747–755 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

