Risk and incidence of fatal adverse events associated with immune checkpoint inhibitors: a systematic review and meta-analysis
- PMID: 30858709
- PMCID: PMC6385768
- DOI: 10.2147/TCRM.S191022
Risk and incidence of fatal adverse events associated with immune checkpoint inhibitors: a systematic review and meta-analysis
Abstract
Background: Given the increasing use of immune checkpoint inhibitors (ICIs), a concomitant rise in adverse events is inevitable. In a recent Phase III trial of ICIs versus placebo, we found the staggering difference of incidence of fatal adverse events (FAEs). Hence, we should determine the risk of FAEs in ICIs.
Objective: To address the risks of FAEs associated with each ICI regimen, we performed a systematic review and meta-analysis of clinical trials with the Food and Drug Administration-approved ICI regimens in patients with advanced solid tumors.
Methods: Literature searching was based on PubMed before April 15, 2018. The numbers of FAEs in both study group and placebo group were collected. We assessed the risk of fatal adverse reactions associated with ICIs on Pooled Peto OR and associated 95% CI.
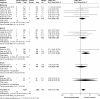
Results: Twelve trials were identified. OR value of FADs in all ICIs was 2.32 (95% CI: 1.33, 4.05; P=0.003). The incidence of FAE in ICI in all included studies were up to 3.2%. OR value of clinical trials of prostate cancer was 3.71 (95% CI: 1.12, 12.26; P=0.03). Among the ICI cohorts, the common FAEs were gastrointestinal toxicity (n=12, 25%), pulmonary toxicity (n=10, 20%), cardiac toxicity (n=5, 10%), and hepatic toxicity (n=5, 10%).
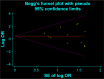
Conclusion: The cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) inhibitors have a significantly higher risk of FAE (P=0.01), whereas programmed cell death protein 1 (PD-1) inhibitors were not. The most common CTLA-4-related FAE was gastrointestinal toxicity, and the most common PD-1-related FAE was pulmonary toxicity. Moreover, we have shown that ipilimumab has significant dose-dependent lethal toxicity.
Keywords: immune mediated death; immune mediated mortality; treatment-related mortality.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Wolchok JD. PD-1 Blockers. Cell. 2015;162(5):937. - PubMed
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. - PubMed
-
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. - PubMed
-
- Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic Chemotherapy-Naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. - PubMed
-
- Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

