A Randomized, Controlled, Phase 2 Study of Maralixibat in the Treatment of Itching Associated With Primary Biliary Cholangitis
- PMID: 30859149
- PMCID: PMC6396374
- DOI: 10.1002/hep4.1305
A Randomized, Controlled, Phase 2 Study of Maralixibat in the Treatment of Itching Associated With Primary Biliary Cholangitis
Abstract
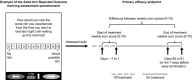
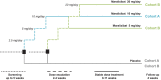
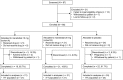
Primary biliary cholangitis (PBC) is typically associated with elevated serum bile acid levels and pruritus, but pruritus is often refractory to treatment with existing therapies. This phase 2 study assessed the efficacy and safety of maralixibat, a selective, ileal, apical, sodium-dependent, bile acid transporter inhibitor, in adults with PBC and pruritus. Adults with PBC and pruritus who had received ursodeoxycholic acid (UDCA) for ≥6 months or were intolerant to UDCA were randomized 2:1 to maralixibat (10 or 20 mg/day) or placebo for 13 weeks in combination with UDCA (when tolerated). The primary outcome was change in Adult Itch Reported Outcome (ItchRO™) average weekly sum score (0, no itching; 70, maximum itching) from baseline to week 13/early termination (ET). The study enrolled 66 patients (maralixibat [both doses combined], n = 42; placebo, n = 24). Mean ItchRO™ weekly sum scores decreased from baseline to week 13/ET with maralixibat (-26.5; 95% confidence interval [CI], -31.8, -21.2) and placebo (-23.4; 95% CI, -30.3, -16.4). The difference between groups was not significant (P = 0.48). In the maralixibat and placebo groups, adverse events (AEs) were reported in 97.6% and 70.8% of patients, respectively. Gastrointestinal disorders were the most frequently reported AEs (maralixibat, 78.6%; placebo, 50.0%). Conclusion: Reductions in pruritus did not differ significantly between maralixibat and placebo. However, a large placebo effect may have confounded assessment of pruritus. Lessons learned from this rigorously designed and executed trial are indispensable for understanding how to approach trials assessing pruritus as the primary endpoint and the therapeutic window of bile acid uptake inhibition as a therapeutic strategy in PBC.
Figures

References
-
- Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. J Hepatol 2015;63:1285‐1287. - PubMed
-
- Poupon RE, Chretien Y, Chazouilleres O, Poupon R, Chwalow J. Quality of life in patients with primary biliary cirrhosis. Hepatology 2004;40:489‐494. - PubMed
-
- Montagnese S, Nsemi LM, Cazzagon N, Facchini S, Costa L, Bergasa NV, et al. Sleep‐wake profiles in patients with primary biliary cirrhosis. Liver Int 2013;33:203‐209. - PubMed
-
- Bergasa NV. The pruritus of cholestasis. J Hepatol 2005;43:1078‐1088. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

